Pain, Disgust, and Analgesia
Electrical stimulation of specific sites of the brain can cause pain, disgust, and analgesia.
It has been confirmed that pain is produced by electrical stimulation of the thalamus and insular cortex, discomfort by that of the insular cortex and limbic system, and analgesia by that of the β-endorphin system.
If abused, this would make it possible to make individuals suffer from unexplained pain that cannot be resolved by doctors.
Table of ContentsAll_Pages
Circuitry
Pain
Non-noxious skin sensations such as touch and pressure are transmitted via the spinothalamic tract to the ventral posterior nucleus (VP) of the thalamus, a sensory relay nucleus. (Craig 2003)
On the other hand, noxious skin sensations such as pain and temperature are primarily transmitted to the region posterior and inferior to the VP, from where they are further transmitted to the insular cortex and, supplementally, to the primary somatosensory cortex.
They are also transmitted to the ventral caudal portion of the medial dorsal nucleus of the thalamus, from where they are transmitted to the anterior cingulate cortex.
Each of these cortical areas is thought to have subfunctions that represent different aspects of pain. (Iannetti and Mouraux 2010)
For example, the sensory aspects of pain are represented in lateral regions of the brain, such as the primary and secondary somatosensory cortices.
On the other hand, the emotional aspects of pain are represented in medial regions of the brain, such as the anterior cingulate cortex.
The insular cortex is the central hub of the pain network, receiving input from both the anterior cingulate cortex and the somatosensory cortices, and has both sensory and emotional functions. (McBenedict et al. 2024 Apr 18)
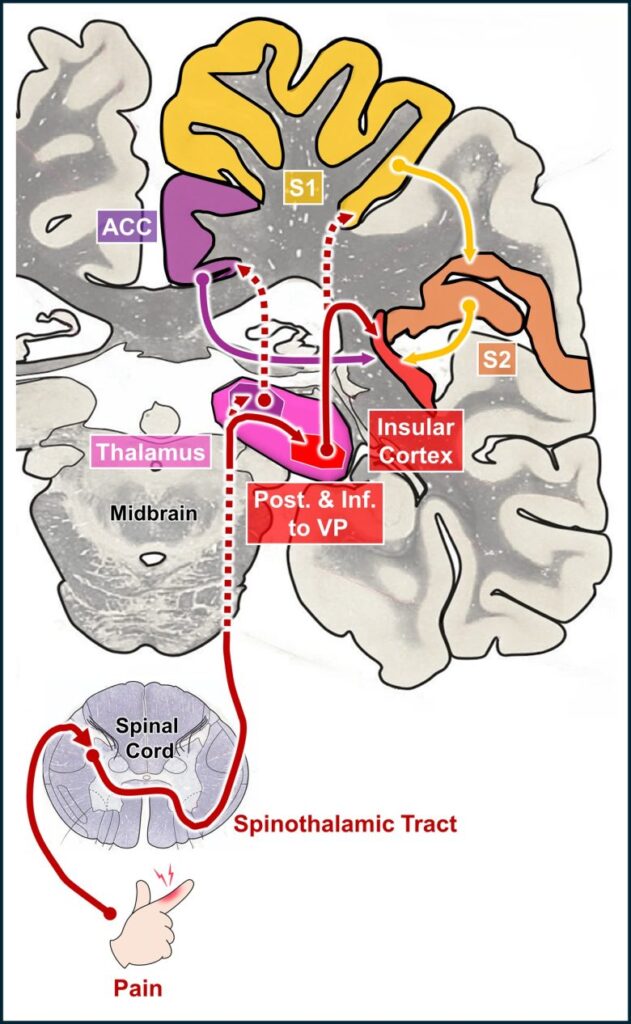
Pain pathways from the peripheral to central nervous systems
(Modified from Craig 2003, the brain image from Vanderah 2018)
VP = ventral posterior nucleus of the thalamus; ACC = anterior cingulate cortex; S1 and S2 = primary and secondary somatosensory cortices.
Of the brain regions described above, it has been confirmed that pain sensations are produced by electrical stimulation of the region posterior and inferior to the VP and its vicinity, and the upper posterior part of the insular cortex and the adjacent secondary somatosensory cortex.
Discomfort
There are two main pathways for transmitting visceral information through the nerves. (Critchley and Harrison 2013)
The first are the fibers that transmit information such as hunger, satiety, thirst, nausea, and respiratory sensations, and travel mainly along the vagus nerve and terminate in the solitary nucleus.
The second are the fibers that transmit information about tissue damage, and travel along the spinothalamic tract and terminate in the thalamus.
Both types of visceral information are relayed to the insular cortex, as well as the limbic system, such as the amygdala and anterior cingulate cortex.


Visceral information pathways from the periphery to the central nervous systems. However, the pathways from the solitary nucleus and the thalamus are simplified. For details, see the source.
(Modified from Critchley and Harrison 2013, the brain image from Vanderah 2018)
ACC = anterior cingulate cortex.
In addition, the insular cortex has also been strongly implicated in perceiving as well as experiencing many forms of disgust. (Chapman and Anderson 2012)
The anterior insular cortex is the area that becomes activated when humans experience disgust. (Wicker et al. 2003)
Electrical stimulation of the anterior insular cortex has been shown to induce visceral discomfort such as abdominal pain, nausea, and upset stomach in humans, and to induce disgust responses against food in monkeys.
Electrical stimulation of the limbic system has also been shown to induce visceral discomfort.
Analgesia
β-endorphin is a neurotransmitter found in the brain that has a morphine-like analgesic effect and acts on both the peripheral and central nervous systems. (Sprouse-Blum et al. 2010)
The β-endorphin system involves cell masses in the basal part of the hypothalamus, which extend through the anterior hypothalamus, septal area, periventricular gray, and toward the periaqueductal gray. (Bloom et al. 1978, Richardson 1982)
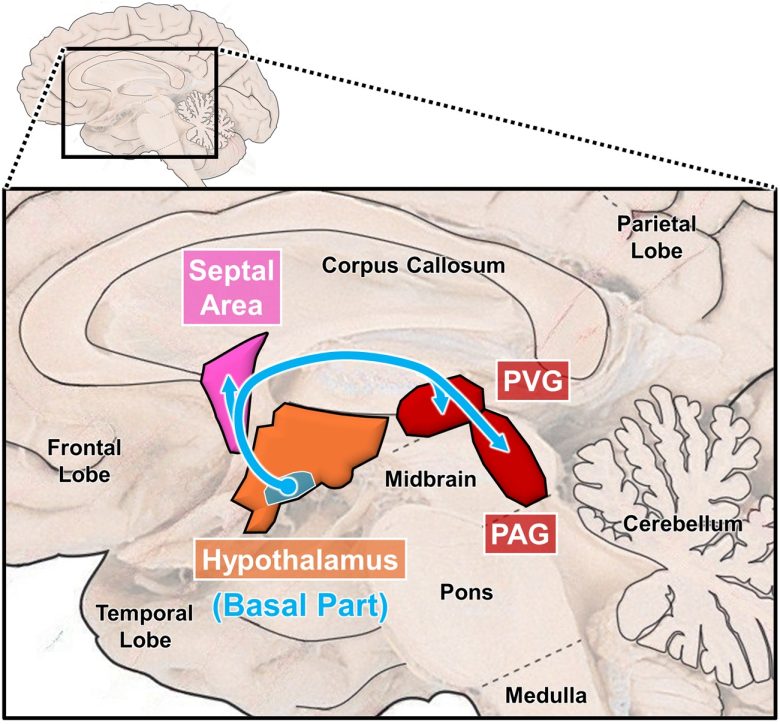
The brain regions of the β-endorphin system
(Modified from Richardson 1982, the brain image from Vanderah 2018)
PVG = periventricular gray; PAG = periaqueductal gray.
Electrical stimulation of these brain regions of the β-endorphin system has been confirmed to produce analgesic effects in both humans and animals.
In particular, excellent analgesic effects have been shown in the septal area and PVG.
Pain
It has been shown that electrical stimulation of the region posterior and inferior to the ventral posterior nucleus (VP), a sensory relay nucleus of the thalamus, and its vicinity can produce pain sensations.
It has also been shown that electrical stimulation of the upper posterior part of the insular cortex and the adjacent secondary somatosensory cortex can produce pain sensations.
Thalamus
German Study
German pathologist Rolf Hassler found that electrical stimulation of the thalamus can produce pain sensations in various parts of the body. (Hassler and Riechert 1959)
Electrical stimulation was performed during thalamic electric coagulation for pain relief, and in all cases pain was obtained from the region inferior to the VP of the thalamus.
At stimulation frequencies of less than 20 Hz, rhythmic twitching in parts of the body occurred in a specific part of the body, but no pain.
If the stimulation frequency was increased, painful paresthesia could occur, and the most severe pain effect was experienced during electric coagulation.
The female patient, Ms. L.O., had syphilis and was suffering from shingles with intense pain on the left side of her face.
Electrical stimulation of the right thalamus at low frequency of 25 Hz produced an inexplicable facial sensation, while stimulation at high frequency of 50 Hz produced a severe pain in the left face.
Electric coagulation of this region produced an extremely painful sensation on the left side of the face for a few seconds, after which an analgesic effect was observed and the patient was completely free of pain.
The female patient, Ms. G.P., suffered from trigeminal neuralgia on the left side of her face.
Electrical stimulation of the right thalamus at low frequency of 1-8 Hz produced twitching at the left upper face, while high frequency of 8-50 Hz produced a pain in the left upper lip and cheek.
Electric coagulation of this region produced a maximum pain, with the patient crying out and distortion of the left side of the face.
The patient subsequently reported that during coagulation, she felt a pain in the left side of her face, extending to the entire left side of her body. She felt as if she were in an ice cellar.
At the end of the operation, the patient was completely free of her pain in the trigeminal nerve region.
A male patient, Mr. B.H., suffered from phantom pain in his left leg following amputation of the left thigh due to an injury.
The main pain sites were on the ball of the foot, in the toes, on the heel, and in the calf, and he felt his toes as if they were digging into the skin of the ball of the foot.
Electrical stimulation of the right thalamus at low intensity caused twitching only of the stump, and as the stimulation intensity increased, also of the left shoulder and the left side of the face.
Electrical stimulation of the thalamus at higher intensity over a few ten seconds produced a phantom limb pain.
Electric coagulation of this region caused a severe pain in the phantom and the entire left side of the body. After three coagulations, the phantom pain of the left leg completely disappeared.
A male patient, Mr. P. G., underwent a right thigh amputation due to a gunshot wound to the knee and gangrene. More than a decade later, when he fractured the remaining thigh bone, he experienced considerable pain in the stump without phantom limb pain.
Electrical stimulation of the thalamus at low frequency of 4 Hz or 8 Hz caused twitching of the stump, the right arm, and the right side of the face in stimulation rhythm.
It also produced an electrifying, non-painful sensation in the right side of the body, particularly in the arm and face.
High-frequency stimulation of 25 or 50 Hz caused a severe pain on the right side of the body, particularly in the right arm with cramping and, to a lesser extent, on the right side of the face, as well as vigorous twitching of the stump.
The patient describes the pain as an electric-like, cramping sensation, not burning, nor does it correspond to the pain he spontaneously experienced in the stump.
Electric coagulation of this region caused an extremely severe pain on the right side of the body, and the patient cried out.
After the ninth coagulation, hypoalgesia to analgesia was observed in the entire right side of the body, including the stump, and there was no longer any spontaneous pain in the stump.
Possibly due to repeated coagulations, the patient suffered hemorrhage in the VP of the thalamus, and died 12 days after the operation due to pulmonary complications.
British Study
At Queen Square Hospital in London, destruction of the thalamus was applied as a treatment for patients with Parkinson's disease. (HALLIDAY and LOGUE 1972)
Prior to the operation, an experiment of electrical stimulation of the thalamus was conducted, and as in the previous study, pain was induced primarily in the region inferior to the VP of the thalamus.

The sensations were described by the patients variously as a localised sharp pain, an ache, or a burning sensation, and were spontaneously distinguished by them from the tingling encountered when stimulating elsewhere.
The threshold intensity for pain was extremely low at 0.01-0.03 mA.
The region from which painful sensations could be evoked appeared to be quite localized, and it was only about 0.08 in (2 mm) in extent, at least for threshold intensities. Above and below this region, only tingling could be evoked.
In the first patient, electrical stimulation of the thalamus produced a burning sensation at the back of the throat.
At a point 0.12 in (3 mm) lateral from there, a tingling sensation occurred in the left arm and fingers, most marked in the thumb and index.
In the second patient, electrical stimulation of the thalamus caused a sensation of a sharp pain in the left wrist.
As the stimulation intensity increased, the pain became more intense, causing the patient to give a little cry.
At a point 0.08 in (2 mm) superior from there, a tingling sensation occurred in the left arm, maximal in the little finger. The patient was quite definite that this was not painful.
In the third patient, electrical stimulation of the thalamus produced tingling in the middle finger. With increasing intensity, it took on a burning quality, and with further increases in intensity, it turned into an unpleasant, burning, and painful sensation.
At a point 0.02 in (0.5 mm) inferior from there, tingling occurred in the middle finger, and as the stimulation became stronger, tingling spread to all four fingers except the thumb. At even stronger stimulation, tingling occurred in all five fingers and the wrist.
In the fourth patient, electrical stimulation of the thalamus caused an ache, mainly in the right little finger, but also in the middle and ring fingers, which was accompanied by a numbness.
As the stimulation intensity increased, the numbing ache spread to the entire hand.
At a point 0.12 in (3 mm) inferior from there, a faint, hot feeling occurred in the right shoulder. With stronger stimulation, the hot feeling spread to the right heel, the back, and the right shoulder.
Canadian Studies
A research group at the University of Toronto also confirmed that electrical stimulation of the region inferior to the VP of the thalamus can produce pain. (Dostrovsky et al. 1992)
The subjects were patients with movement disorders or chronic pain who were scheduled for destruction of the thalamus, and an experiment of electrical stimulation of the thalamus was conducted prior to the operation.
In addition to pain sensations, warm and cold sensations were obtained in a limited region inferior to the VP of the thalamus.
The sensations occurred at the opposite side of the stimulation and usually were limited to a discrete part of the body.
The pain was either described in terms of natural pain sensations such as burning or needle insertion or in terms of abnormal but painful sensations such as electric shock.
Electrical stimulation of the VP itself usually produced only non-painful skin sensations.
However, in the pain patients, the pain incidence outside the region inferior to the VP increased.
In one patient, the sites of the body where pain sensations occurred shifted as the electrode was advanced: as the electrode position moved from top to bottom, the skin representation shifted from bottom to top: fingertip → lower arm → upper arm → shoulder.
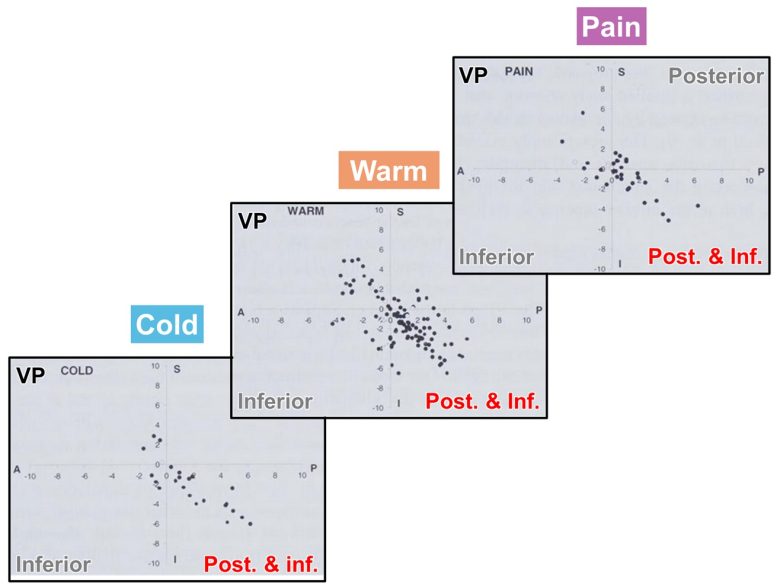
The research group subsequently investigated in detail the sites in the thalamus that induce pain sensations and warm and cold sensations on a large group of 86 patients. (Dostrovsky et al. 2000)
They found that most sites were concentrated in the region 0.04-0.12 in (1-3 mm) posterior and inferior to the VP.
In some cases, pain sensations and warm and cold sensations were induced from the VP itself.
In addition, patients with central pain had a marked increase in pain incidence on stimulation compared with patients with peripheral pain or movement disorders.
American Studies
At Johns Hopkins Hospital, an experiment of electrical stimulation of the thalamus was also conducted on patients with movement disorders and chronic pain. (Lenz et al. 1993)
The results were generally similar to those of the Canadian study, with pain sensations and warm and cold sensations coming primarily from the region posterior and inferior to the VP of the thalamus.
For chronic pain patients, a pain incidence markedly increased in the body parts where patients experience chronic pain.
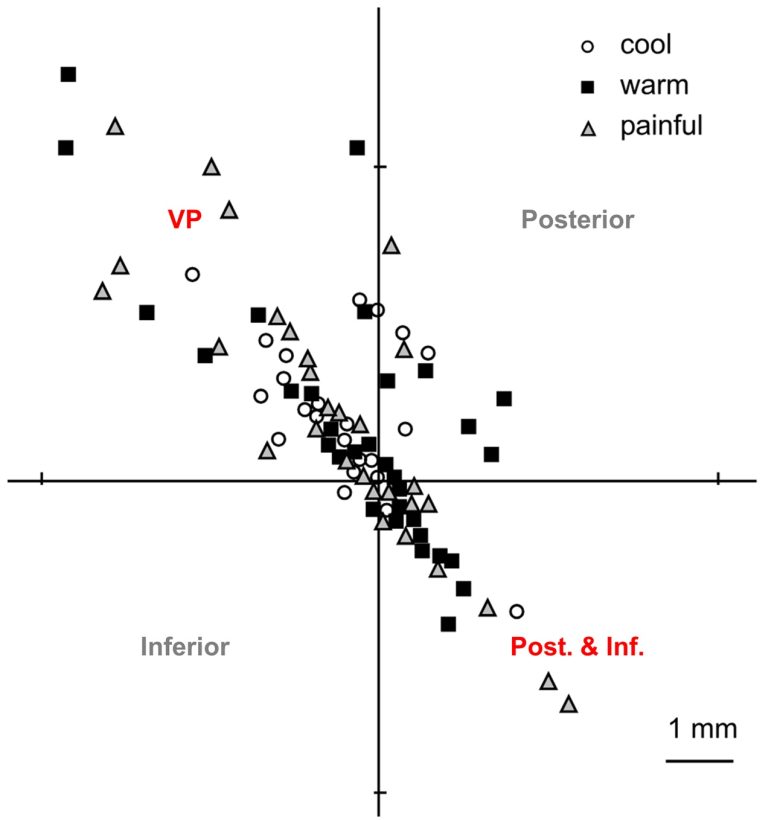
A subsequent study at the same hospital on a larger group of 124 patients yielded slightly different results. (Ohara and Lenz 2003)
This experiment showed that pain sensations and warm and cold sensations could be induced to the same extent not only from the region posterior and inferior to the VP, but also from the VP itself.
Spinothalamic Tract
At Duke University Medical Center, destruction of the midbrain tegmentum was applied as a treatment for patients with intractable pain. (Nashold et al. 1969)
Prior to the operation, an experiment of electrical stimulation of the midbrain tegmentum was conducted.
Electrical stimulation of the lateral region of the tegmentum produced an intense pain, as well as numbness, electrifying sensations, and burning sensations.
The pain was felt on the opposite side of the body from the stimulation.
Electrical stimulation of the medial region of the tegmentum also produced an intense pain, which was, however, felt in the central parts of the body, such as the head, neck, chest, and abdomen.
Some of these sensations are felt within the oral cavity, involving the tongue, gums, and teeth, and one man experienced a metallic taste with his oral sensation.
If the sensation was associated with the chest it was often felt in the region near the heart.

It was speculated that the pain was caused by activation of pain-related nerve fibers, such as those in the spinothalamic tract, which pass through the midbrain tegmentum.
Insular Cortex
French Studies
At the Pierre Wertheimer Hospital in Lyon, many experiments of electrical stimulation of the insular cortex have been conducted on patients with epilepsy.
A research group at the hospital showed for the first time that electrical stimulation of the insular cortex can induce pain. (Ostrowsky et al. 2000)
The subjects were 14 epilepsy patients, and electrical stimulation of the posterior insular cortex induced skin sensations, including pain.
Skin sensations included warmth sensations in the arm or leg, tingling in the nose or arm, and once the sensation that the “skin was being separated from the underlying tissues” on both hands simultaneously.
The pain sensations were painful electrical shock or painful tingling, and occurred in the hand or arm on the side opposite to the stimulation.
On the other hand, electrical stimulation of the anterior insular cortex induced visceral sensations and visceral movements.
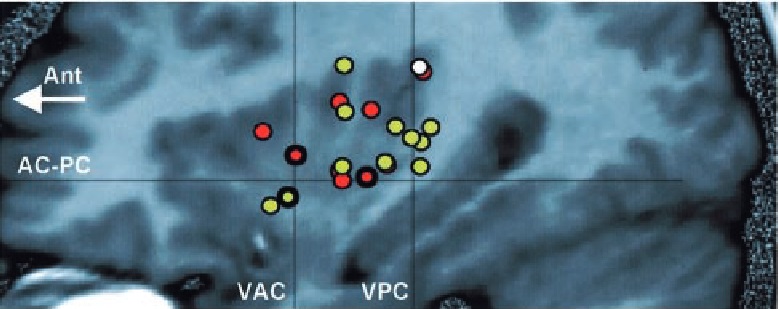
A subsequent study broadened the patient population from 14 to 43 patients and focused on skin sensations induced by electrical stimulation of the insular cortex. (Ostrowsky 2002)
They found that pain sensations primarily occurred in the upper posterior part of the insular cortex.
On the other hand, non-painful skin sensations occurred widely in the posterior part of the insular cortex.
Both sensations occurred on the side opposite to the stimulation, and on both sides if near the midline of the body.
Qualities of the evoked pain were described as burning, stinging, or disabling sensations. Electrical shocks or discharges were also reported.
Non-painful skin sensations were reported as warm, cold, or tingling sensations.

Red = electrical shock; orange = burning sensation; white = stinging sensations; blue = disabling sensations.
Green = tingling sensations; red = warm sensations; white = cold sensations.
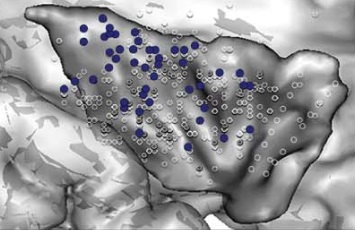
Recently, they analyzed the results of electrical stimulation of the insular cortex obtained from an even larger patient population of 222 patients. (Mazzola et al. 2017)
The responses obtained from the insular cortex were diverse, including skin sensations, visceral sensations, auditory sensations, vestibular sensations, speech impediments, gustatory sensations, and olfactory sensations.
Skin sensations were obtained from the posterior part of the insular cortex and accounted for 60% of all evoked sensations, including paresthesiae, thermal sensations, and pain.
Non-painful skin sensations were reported as neutral or unpleasant sensations of tingling, light touch, or slight electric current.
Pain sensations were mostly obtained from the upper posterior part of the insular cortex.
All patients who reported a painful sensation also had spontaneous behavioral manifestations of pain, including facial expressions or verbal complaints.
Qualities of the evoked pain were described as burning, painful sensations of electricity or electric shock, stinging, painful pins and needles, and crushing or cramp sensations.
The posterior part of the insular cortex had body-part representation of pain, with the face area being ahead of the upper limb area, and the latter being above the lower limb area.
At the Grenoble Alpes University Hospital, France, an experiment of electrical stimulation of the insular cortex was conducted on 25 epileptic patients. (Afif et al. 2008)
Pain responses occurred in 5 patients.
Half of the responses occurred in the head, and the other half in the upper limbs, shoulders, and trunk. The latter felt like pinprick sensations.
This experiment showed that most of the pain occurred in the middle short gyrus (anterior region) of the insular cortex, a finding that differs from other studies.
In addition, stimulation of the posterior part of the insular cortex induced non-painful sensations of warmth in four patients.

American Study
UH Cleveland Medical Center conducted an experiment of electrical stimulation of the insular cortex on five epileptic patients. (Stephani et al. 2010)
As a result, skin sensations, visceral sensations, and gustatory sensations occurred in three patients.
Skin sensations were obtained primarily from the posterior part of the insular cortex.
Painful sensations were obtained from a region restricted to the upper posterior part of the insular cortex and were described as "burning," "sting-like," or simply "painful."
Warm sensations were described as a "warm" or "hot" feeling.
Not-painful skin sensations were described as "tingling," "feeling of pulsation," "feeling of vibration," or "feeling of numbness."
The skin sensations always occurred on the side opposite to the stimulation.
Secondary Somatosensory Cortex
French Studies
The research group at the Pierre Wertheimer Hospital discovered that pain can be induced not only by electrical stimulation of the insular cortex but also by that of the secondary somatosensory cortex. (Mazzola et al. 2005)
The subjects were 14 patients with epilepsy, and in three patients, pains of mild to intolerable intensity were induced.
The first patient felt painful tingling in the superior part of the face, predominating on the left side.
The second patient reported a painful cramp and an electric current sensation on the left half of his body.
The third patient felt a painful cramp and tingling in his left cheek spreading rapidly to the left half of his body.
Electrical stimulation of the secondary somatosensory cortex induced pain at a rate similar to that of the insular cortex, but that of the primary somatosensory cortex did not induce pain.
Body parts represented in the insular cortex tended to be larger than those in the secondary somatosensory cortex, and body parts represented in the secondary somatosensory cortex tended to be larger than those in the primary somatosensory cortex.
The research group investigated the regions throughout the cerebral cortex where pain was induced by electrical stimulation. (Mazzola et al. 2011)
In the experiment, they analyzed the results of electrical stimulation of various cortical areas obtained from a large patient population with epilepsy of 164 patients.
They found that the pain-inducing sites were concentrated only in the medial part of the secondary somatosensory cortex and the upper posterior part of the insular cortex, which are topographically contiguous.
Other cortical areas didn't induce pain.
In both regions, the terms used by the patients for describing qualities of their pain were similar: burning, painful sensations of electricity or electric shock; stinging, painful pins and needles; and crushing or cramp sensations.
Pain intensity was similar in both regions as well.
All patients who reported a painful sensation also had spontaneous behavioral manifestations of pain, including facial expressions of pain, verbal complaints including shouts and cries, movements to avoid the stimulation, or autonomic changes such as facial pallor or redness.
Pain was most often on the opposite side to the stimulation but could also be on the same side or on both sides when the pain concerned parts of the body close to the midline, such as the face or trunk.
Discomfort
Electrical stimulation of the anterior part of the insular cortex has been shown to produce visceral discomfort, and in an experiment with monkeys, electrical stimulation of this region produced disgust responses against food.
Electrical stimulation of the limbic system, including the amygdala, anterior cingulate cortex, and hippocampus, has also been shown to produce visceral discomfort.
Insular Cortex
Canadian Study
Dr. Wilder Penfield, a neurosurgeon at the Montreal Neuroscience Institute, discovered that electrical stimulation of the insular cortex can produce visceral and skin sensations. (PENFIELD and FAULK 1955)
The visceral sensations were variously described by the patient as "something funny" referred to the abdomen, also "gurgling," "rolling," "pain," "nausea," "scratching," or simply as "sensation."
The skin sensations were variously described by the patients as "tingling," "warmth," "numbness," "tightness," "vibration," "shock," and simply as "sensation."
French Studies
The research group at the Pierre Wertheimer Hospital in Lyon confirmed these findings in an experiment on 14 epilepsy patients. (Ostrowsky et al. 2000)
Visceral sensations were produced by electrical stimulation of the anterior part of the insular cortex, and skin sensations were produced by electrical stimulation of the posterior part of the insular cortex.
Visceral sensations were described as nausea or as a pressure in the upper abdomen or a throat sensation, with once a very unpleasant strangling sensation.
Other responses observed included gustatory, auditory, olfactory, and visual hallucinations, and speech disturbances.
The research group also showed that visceral sensations obtained from the insular cortex are associated with disgust. (Krolak‐Salmon et al. 2003)
In the experiment, they studied 13 epilepsy patients to investigate the regions of the insular cortex that were activated when they viewed human facial emotional expressions of fear, disgust, happiness, surprise, and neutral expressions.
They found that, in four patients, the anterior part of the insular cortex became activated when they viewed a facial expression of disgust.
Electrical stimulation of this same region produced unpleasant visceral sensations and paresthesia.
Two of them reported unpleasant sensations in the throat spreading up to the mouth, lips, and nose. It was not painful but described as “difficult to stand.”
The third patient reported paresthesia in the hand of the opposite side when the deepest electrode contact was stimulated. This contact was not lying in the insular cortex, but in the underlying white matter.
Recently, they analyzed the various responses by electrical stimulation of the insular cortex obtained from a large patient population of 222 patients. (Mazzola et al. 2017)
Visceral sensations were the second most common response induced after skin sensations, and were obtained from a more anterior region than the region where skin sensations were obtained.
What was mainly produced were unpleasant sensations of constriction in the throat, chest, and abdomen, varying in severity from a simple discomfort to a frightening sensation of strangulation.
Autonomic symptoms also occurred, such as nausea, salivation, facial blush, fainting, shortness of breath, urge to urinate, and sweaty hands.
Other than that, constrictive sensations in the chest or abdomen were sometimes accompanied by feelings of anxiety and fear.
It ranged from mild anxiety to real panic, and in some cases, anxiety alone was present.
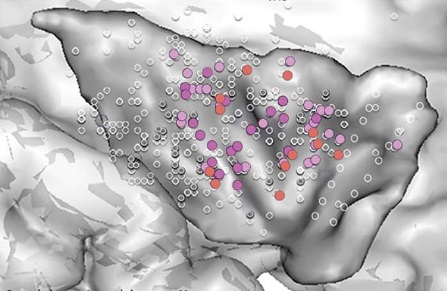
Light pink = constrictive sensations; deep pink = autonomic symptoms; orange = mental symptoms.
American Study
UH Cleveland Medical Center conducted an experiment of electrical stimulation of the insular cortex on five patients with epilepsy. (Stephani et al. 2010)
In three patients, skin sensations, visceral sensations, and gustatory sensations were obtained.
Visceral sensations were obtained from a more anterior region than the region where skin sensations were obtained.
The visceral sensations were described as a feeling of "throwing up," having "something in the throat," "vibration in the stomach," or simply "abdominal sensation."
The gustatory sensations were induced from the central part of the insular cortex.
Often these were unpleasant phenomena described as "bad," "nasty," or "nauseous" tastes, and were sometimes qualified as "metallic" or "like aluminum."
Italian Study
Researchers at the University of Parma in Italy conducted an experiment of electrical stimulation of the insular cortex on monkeys. (Caruana et al. 2011)
Electrical stimulation of the anterior part of the insular cortex in two rhesus monkeys produced disgust responses.
The effects of electrical stimulation were tested during two different moments of spontaneous feeding: while the monkey was bringing food to the mouth, and during chewing.
In both instances, the monkey refused the food.
In the first one, when the stimulation started while food was in its hand, the monkey threw the food away immediately, even if the food was the one the monkey liked most.
In the latter one, when the stimulation started with the food already inside the mouth, the monkey spat it out.
Limbic System
American Study
Dr. John Van Buren of the National Institutes of Health studied abdominal sensations obtained by electrical stimulation of the temporal lobe on epileptic patients. (Van Buren 1963)
The sensations caused by the stimulation were sometimes accompanied by actual stomach peristalsis (involuntary digestive movements).
Patient W.A. received electrical stimulation of the left hippocampus.
It produced a "sick feeling" in his stomach and repeated swallowing. His face became flushed and he did not respond immediately. The nausea persisted for 5-10 minutes after the stimulation.
The sick feeling was accompanied by vivid thoughts, and he remarked that he had thought about a bridge over a stream near his home.
In another session, after the electrical stimulation of the same site, the patient stated that he felt sick to his stomach. Five seconds later he ceased breathing, and became unresponsive. After 10 seconds, bradycardia appeared, with the pulse rate falling from 92 to 48 beats per minute.
Subsequently, following an automatism lasting two minutes he passed into an epileptic seizure.
Patient M. B. received electrical stimulation of the right amygdala.
He reported, "Feel like a seizure anytime—a feeling in my stomach."
He then added, "Some odor in the air I can taste." When questioned as to its nature he replied, "No, it's not a bad odor."
Patient R.G. received five electrical stimulations to the same site of the right amygdala.
In three instances a sensation was reported as "a sick feeling like before an attack," "a sick feeling through my nose," and "a new sick feeling through my stomach."
Other sensations reported were "vibration in my nose" and "quivering in my left thigh."
Patient G.L. received electrical stimulation of the right hippocampus.
Nine out of ten times, stimulation produced a sensation in the upper abdomen.
She described the sensation as being in her "stomach," calling it "butterflies." She also reported a "dizzy" sensation with each stimulation.
During this period stomach peristalsis appeared gradually, becoming more regular and of greater amplitude.
Patient Mr. G. T. received electrical stimulation of the right hippocampus.
Stimulation caused him to report, "Now my stomach feels funny," which was accompanied by slowing breathing, and esophagus peristalsis.
On subsequent stimulation he said, "Now I feel it in my stomach. It felt like it was getting big. Just before that I felt real light-headed."
French Studies
At the Grenoble Alpes University Hospital, France, a comprehensive mapping of the brain regions that induced visceral sensations was carried out. (Mulak et al. 2008)
In the experiment, they analyzed the results of electrical stimulation of various brain regions obtained from a large patient population with epilepsy of 339 patients.
They found that many visceral sensations were obtained from the insular cortex and its overlying operculum as well as from the limbic system.
Visceral sensations included a warm sensation, discomfort, squeezing, pain, nausea, an electric shock-like sensation, and a gustatory hallucination, with pain occurring in the stomach and esophagus.
Other descriptions were also reported, including a sensation of a knot in the stomach or an empty stomach, heaviness, a vague sensation, a sensation of insects crawling, and stinging or tickling.
These responses occurred most frequently in the upper abdomen, followed by the chest, throat, mouth, and lower abdomen.
Depending on the stimulation site, the visceral area where sensation occurred varied.
The regions that induced the upper abdomen sensations were, in order of frequency, the temporal pole, hippocampus, anterior cingulate cortex, and amygdala.
The chest sensations were most frequently induced from the anterior cingulate cortex.
The stimulation of the anterior cingulate cortex provoked chest pain, possibly related to the esophagus constriction.
The mouth and throat sensations were most frequently induced from the insular cortex and its overlying operculum.
The stimulation of the insular cortex induced a throat sensation characterized as an unpleasant constriction, often with paresthesia in the throat.
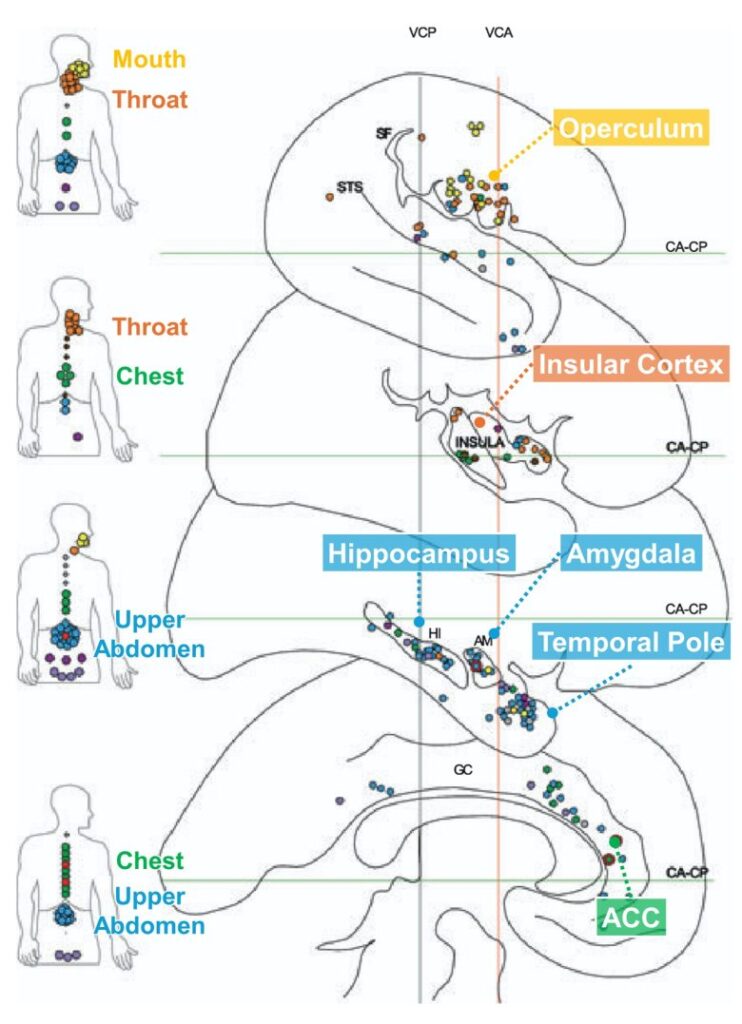
In the insular cortex, throat and chest sensations are common.
In the amygdala, hippocampus, and temporal pole, upper abdomen sensations are common.
In the anterior cingulate cortex (ACC), chest and upper abdomen sensations are common.
These visceral sensations were sometimes accompanied by anxiety, salivation, swallowing, difficulty to talk, voice changes, flushing, tachycardia, pulsation in the head, paresthesia, goosebumps, shivers, vertigo, visual problems, or a feeling of unreality.
The research group at the Pierre Wertheimer Hospital in Lyon investigated the link between visceral sensations and emotional responses in the brain. (Soulier et al. 2022)
In the experiment, they analyzed the results of electrical stimulation of various brain regions obtained from a large patient population with epilepsy of 203 patients.
They found that visceral sensations and emotional responses are induced from common regions: the insular cortex/operculum and the limbic system.
The visceral sensations were localized in the abdomen, chest, and throat, and the patient experienced abdominal pain, sensations in the upper abdomen, abdominal discomfort, throat constrictions, and chest sensations.
These were most frequently obtained from the amygdala, followed by the insular cortex, operculum, hippocampus, and parahippocampal gyrus.
Emotional responses consisted of a feeling of anxiety, fear, or sadness, which were elicited more frequently in women. No positive feeling or mirth was elicited.
These were obtained primarily from the amygdala, anterior cingulate cortex, and hippocampus, but also from the anterior part of the insular cortex and the frontal operculum.
Interestingly, half of emotional responses evoked by stimulations were associated with visceral sensations such as sensations in the upper abdomen, throat and chest constrictions, abdominal discomfort, abdominal pain, flush, and nausea.
Analgesia
Electrical stimulation of the brain regions of the β-endorphin system, such as the septal area, periventricular gray (PVG), and periaqueductal gray (PAG), has been shown to produce analgesic effects.
Septal Area
Humans
Psychiatrist Robert Heath of Tulane University found that pain patients who received electrical stimulation of the septal area experienced relief from severe pain. (Heath and Mickle 1960)
The subjects were six intractable pain patients, four with far advanced metastatic cancer and two with severe rheumatoid arthritis, who had failed to achieve pain relief with medication.
The effect of electrical stimulation of the septal area was quite startling.
They experienced immediate pain relief and felt good, smiled, brightened up, and changed their facial expressions.
The effect of a single electrical stimulation lasted for a maximum of 2-3 days.
One rheumatoid patient received a long-lasting effect. He was stimulated a total of 3 times and did not have severe pain from then on.
Some discomfort persisted because of osteoporosis, but he had no persistent hot, swollen, red joints.
The other rheumatoid patient had a lot of contractures; she had not been able to move for quite some time.
During the stimulation her joints loosened up and she could pump, like riding a bicycle.
However, her effects were of brief duration. They would last for the greater part of the day and then she would have to be restimulated.
Spanish neurophysiologist Jose Delgado also showed that electrical stimulation of the septal area can produce analgesia. (Delgado et al. 1972, Obrador et al. 1974)
One male patient had the left brachial plexus damaged in a car accident, resulting in paralysis in the left arm and a phantom limb appeared causing intolerable pain.
Upon electrical stimulation of the septal area, marked relief of pain was rapidly observed and his pre-operative hostile reaction toward the nurses and other patients disappeared.
The electrodes were placed chronically for more than a year and a half, and electrical stimulation was administered 3-5 times a week.
The patient experienced no discomfort from the device and was able to live at home, return to work, and reintegrate into society.
Another male patient suffered from post-traumatic facial pain and had tried various treatments without success.
This patient also received pain treatment with continuous electrical brain stimulation.
Electrical stimulation of the centromedian-parafascicular nuclei complex of the thalamus induced very short-lasting pain relief.
Electrical stimulation of the pulvinar nucleus induced marked diminution of pain during several hours.
Electrical stimulation of the septal area induced emotional changes of well-being and relaxation, and gave pain relief for periods of up to 18 hours.
With combined septum and pulvinar stimulation pain relief would sometimes last 2 days.
One female patient suffered from advanced cancer and widespread pain requiring daily several morphine injections.
She also received continuous electrical brain stimulation for pain treatment for three months before she died of cancer.
Electrical stimulation of the septal area induced a feeling of well-being and relaxation that sometimes lasted several hours.
Electrical stimulation of the centromedian nucleus of the thalamus produced a short-lasting abolition of the pain and a feeling of emptiness of a pleasant nature, together with a tendency to sleep.
Combining stimulations of the centromedian nucleus and septal area, the pain could sometimes be controlled for periods of 1 or 2 days.
These brain stimulations, along with mild analgesics, were sufficient to relieve the severe pain of the terminal cancer patient.
At a Veterans Administration medical facility in Texas, electrical stimulation of the septal area was administered to six terminal cancer patients for pain treatment. (Gol 1967)
One case showed complete relief of pain, and the other showed partial relief.
A 43-year-old man was in severe intractable pain from metastatic cancer, probably originating from lung cancer.
When the electrical stimulation of the septal area was started, an immediate response was noted. The patient stated that he felt no pain, and while being stimulated, he felt comfortable.
In addition, the patient appeared to be much more alert. He was able to smile and converse lucidly.
When the patient was on the stimulator, he was alert and active when awake, talking clearly, fully oriented, cheerful and complaining of no pain at all.
He could also rest satisfactorily at night.
Stimulation was continued every other day for approximately three weeks, during which period the patient repeatedly obtained marked pain relief.
No narcotics were required until the patient died of pneumonia due to his neoplastic disease.
No effect was seen in the other four patients, but it is important to note that this study used a rather unusual stimulation method, with frequencies ranging from 2000 to 5000 Hz.
A researcher at the University of Buenos Aires succeeded in very long-term pain relief for up to 10 years by electrical stimulation of the septal area. (Schvarcz 1993)
The subjects were 19 patients with neurogenic pain, and 12 of them showed pain relief with electrical stimulation of the septal area.
Six people had significant pain relief of more than 75% and six people had moderate pain relief of 50-75%.
The follow-up periods ranged from 0.5 to 10 years.
Stimulation with somewhat higher intensity produced a sensation of warmth and often also a feeling of well-being and relaxation.
Animals
A study at the University of Notre Dame showed that electrical stimulation of the septal area can produce pain relief in rats. (Breglio et al. 1970)
First, they placed a bar that delivers electrical stimulation of the septal area in the cage, and had the rats learn to self-stimulate their brains.
Next, the rats were given electric foot shocks while they were self-stimulating and while they were not, and the startle responses they displayed were compared.
They found that when the rats were self-stimulating the septal area, the number of startle responses to foot shock was markedly decreased, and the magnitude of startle responses to foot shock also decreased.
The test was carried out over several days, but from the third day onwards, the rats developed a tolerance to the foot shock, and the effect of electrical stimulation of the septal area on pain relief could no longer be confirmed.
Decrease in Pain Responses
PAG
Animals
Stanford Research Institute (now SRI International) found that electrical stimulation around the periaqueductal gray (PAG) in rats produced a powerful analgesic effect. (Reynolds 1969)
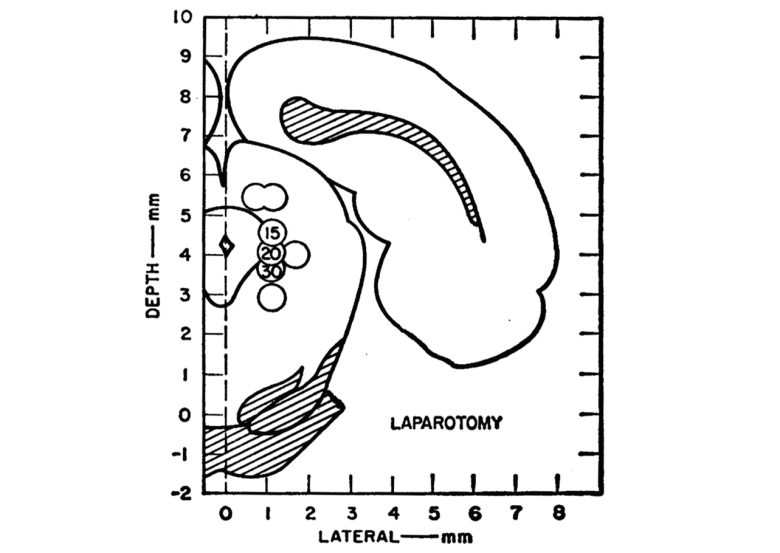
Of eight rats that received electrical stimulation around the PAG, three lost their aversive responses to pressure by forceps on the paws.
Next, these three animals underwent open surgery with brain stimulation.
During the operation, the rat was secured lying on its back.
Abdominal skin, muscle, and peritoneum were sectioned and retracted sufficiently to permit visualization of the abdominal cavity.
This was followed by closure of the incisions with wound clips.
None of the surgical procedures evoked aversive reactions.
Following the operation, brain stimulation was discontinued. Some residual skin analgesia was noted for several minutes, but vigorous aversive responses to forceps-applied pressure returned fully within 5 minutes.
In response to this, a UCLA research group searched extensively from the brainstem to the diencephalon in rats for sites of analgesic effects. (Mayer et al. 1971)
They found that the sites that produce pain relief are located in wider regions, including the midbrain tegmentum, thalamus, and hypothalamus.
However, the highest concentration of electrodes providing analgesia was in the PAG, reinforcing the previous study.

Stimulation at 7 of 19 electrode contacts resulted in complete analgesia to electric shock to the tail.
Even tail shocks which caused tissue damage elicited no observable responses when administered during or shortly after brain stimulation.
In addition, stimulation at 3 of 23 electrode contacts resulted in complete analgesia of at least one limb or the tail to the intense pinch with needle-nose pliers.
Responsiveness to other forms of noxious stimuli was tested in the four rats shown to be unresponsive to pinch.
A heat stimulus, left in contact with the skin long enough to produce blistering, evoked no response from these rats.
Similarly, standing in ice water gave no apparent discomfort.
One rat, for example, ate food pellets for over 5 minutes while standing on its hind legs in a shallow trough of ice water. Approximately 30 seconds after brain stimulation was turned off, the rat suddenly dropped a pellet and jumped out of the trough.
The research group showed that, in cats alike, electrical stimulation of the PAG produced a powerful analgesic effect. (Liebeskind et al. 1973)
Of the 26 electrode contacts tested, stimulation of 11 resulted in a clear and powerful analgesia.
During the electrical stimulation of the PAG, pinches were applied to the four limbs, the tail, and the ears of the cats with forceps.
No behavioral manifestations of withdrawal or aversion were seen in the cats, even during the application of intense pinches, which, in the normal cat, would have provoked vigorous escape and defensive reactions.
In contrast to that seen in the rats, analgesia in these cats rarely outlasted brain stimulation by more than several seconds and never by longer than 1 minute.
The remaining 15 electrode contacts could not be adequately tested for analgesic effects due to the elicitation of strong motor responses and emotional responses like escape and attack.
Humans
Based on the animal findings, Louisiana neurosurgeon Dr. Donald Richardson tested the analgesic effects of electrical stimulation of the PAG on humans. (Richardson and Akil 1977)
The patients scheduled for destruction of the thalamus for pain treatment were selected as the subjects.
A 65-year-old male patient had undergone right leg amputation due to diabetic gangrene and subsequently suffered from severe phantom limb pain.
His phantom limb pain occurred at the site that had been painful prior to the surgery.
Electrical stimulation of the PAG produced excellent chronic pain relief with a general sensation of well-being and relaxation.
The analgesic effect lasted for approximately 10 minutes after 1 minute of stimulation.
A 63-year-old female patient suffered from intractable pain in the right brachial plexus due to breast cancer.
The patient received electrical stimulation of the PAG as well as the posterior medial thalamus and pulvinar nucleus, at all of which sites she reported good-to-very good pain relief.
The analgesic effect lasted for up to 30 minutes after 2 minutes of stimulation.
A 66-year-old male patient suffered from thalamic syndrome and intention tremor following a stroke. The patient complained of intense pain during moving the right arm and leg.
Electrical stimulation of the PAG produced excellent analgesia.
Chronic pain was abolished, and complete analgesia to pinprick was observed. This reduction involved areas of the chest and face, as well as the limbs and lasted about 3-5 minutes.
Overall, electrical stimulation of the PAG was certainly effective in relieving chronic pain, but it also produced numerous undesirable side effects, including nystagmus, nausea, vertigo, and a feeling of a "rising vapor."
PVG
Humans
As an alternative to periaqueductal gray (PAG) stimulation, which has noxious side effects, Dr. Richardson evaluated the pain relief obtained from electrical stimulation of the periventricular gray (PVG). (Richardson and Akil 1977)
Eight pain patients were selected as the subjects and underwent long-term electrical stimulation of the PVG over a period of more than two years.
The results were very encouraging, with six rated as good, one as fair, and one as poor.
A 31-year-old male patient suffered from chronic severe pain in the leg and low back, resulting from lumbar disc disease, and all previous attempts at pain relief had failed.
He had been unable to continue his work as a house electrician for 2 years due to this pain.
Electrical stimulation of the PVG provided total pain relief.
He used the stimulator for 20-30 minutes at bedtime, and pain relief lasted up to 24 hours.
After his surgery, he had several interim jobs involving hard physical work, such as laying bricks. He then obtained a stable position as an electrician.
Pain seemed to increase during stressful times, such as a death in the family or the loss of employment, and he frequently used the stimulator during these times.
A 63-year-old man had recurrent colon cancer and had undergone a rectum resection and had an artificial anus constructed.
He suffered from intractable perineal pain, spreading to the back and the legs, which he described as deep and extremely strong. The "tenderness" prevented him from lying on his back.
Electrical stimulation of the PVG significantly provided very good pain relief, which allowed him to rest on his back for the first time in months.
No medication was no longer needed at all, and a single 20-minute electrical stimulation resulted in 4-5 hours of pain relief.
He described the pain as "floating away."
If high current levels were used, the patient reported a startle reaction, some dizziness, exhibited some nystagmus, and felt frightened and apprehensive.
At the level he typically used, however, the only side effect was a warm sensation down his back, which he perceived as quite pleasant.
He died 2 months after the surgery as a result of ureteral obstruction and uremia, during which period there was no apparent decrement in the effectiveness of brain stimulation.
A 24-year-old woman had suffered a right brachial plexus avulsion during a car accident.
Her arm was atrophic and hypersensitive, and she had continuous dull pain, which became acutely exacerbated by any sensory stimulus, rendering it "focused" and intense.
Electrical stimulation of the PVG makes her arm feel "just like the other."
The relief effect was excellent, lasting for 24 hours with just a few ten minutes of electrical stimulation.
She was able to use her right arm to help carry or support her newborn daughter and various objects, without feeling pain.
During stimulation, she experienced some tingling in her face and a "cold sensation." However, the overall feeling is one of "well-being and relaxation."
The patient became much more cheerful and active after her surgery.
β-endorphin System
The β-endorphin system involves cell masses in the basal part of the hypothalamus, which extend through the anterior hypothalamus, septal area, periventricular gray (PVG), and toward the periaqueductal gray (PAG). (Bloom et al. 1978, Richardson 1982)
Humans
Dr. Richardson evaluated the analgesic effects of the β-endorphin system in the brain region in nine pain patients. (Richardson 1982)
The hypothalamus, septal area, PVG, and PAG were selected as stimulation sites.
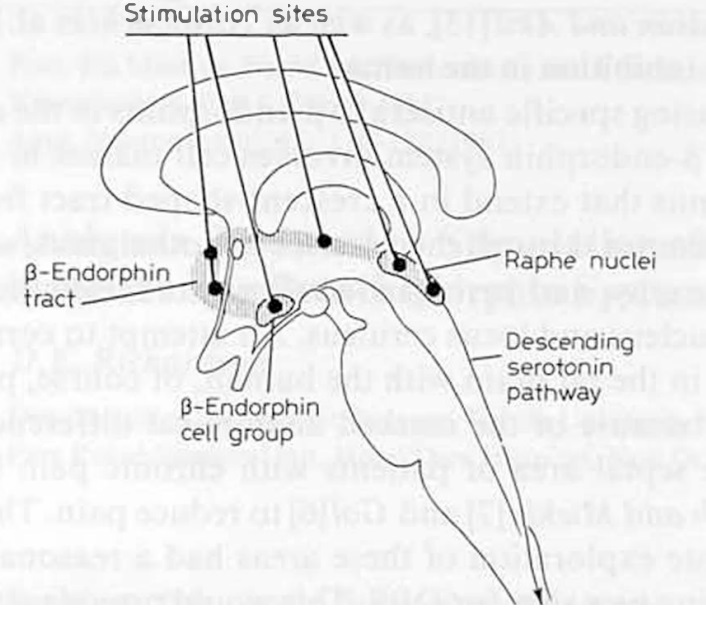
In eight out of nine patients, electrical stimulation of any of the brain regions in the β-endorphin system was able to reduce pain.
The hypothalamus was tested in one patient, and they experienced pain relief.
Other sites were tested in five patients, and four experienced pain relief at all sites.
Stimulation was particularly effective in the septal area and PVG, both of which gave significant pain relief with relatively mild or minimal side effects.
The side effects tended to increase in the hypothalamus and also in the PAG, such as oscillopsia, conjugate deviation, paresthesia, vertigo, flushing, tingling, strabismus, pinprick, smothering, and diplopia.
All patients, except one who did not experience pain relief, chose to have the electrodes left in place for continuous electrical brain stimulation.
Three patients had the electrodes left in the septal area, four in the PAG, and one in the internal capsule. The remaining patient chose the PVG.
Researchers at UC San Francisco administered electrical stimulation of the PAG and PVG to six patients with intractable pain. (Hosobuchi et al. 1977)
Five patients reported total relief and one reported partial relief from pain.
Those who reported total relief from their pain rated their pain level as 0-1 on the 0-10 scale and completely eliminated their intake of analgesic drugs.
In the absence of brain stimulation an average pain level of 6-8 was usually reported, even with an intake of intramuscular morphine.
In addition to effective control of pain in the absence of analgesic drugs, all patients noted marked enhancement of their general feelings of well-being, increased appetite, and an ability to sleep better without sleep medication.
In three patients, the pain relief provided by electrical stimulation diminished rapidly within four to five weeks, even when the output was increased to the limit of tolerance.
That is, tolerance to electrical stimulation to the brain had developed, and electrical stimulation even caused tolerance to narcotics.
After the patients limited the stimulation to no more than 1-2 hours at a time and waited at least 3-4 hours between periods of stimulation, tolerance to both the stimulation and the narcotic no longer occurred.
UCLA researchers evaluated the pain relief effects of electrical stimulation of the PAG, PVG, thalamus, and internal capsule on 48 patients with intractable pain. (Young et al. 1985)
In the subjective evaluation by the subjects, 16 considered that their pain had been completely relieved, 19 thought that at least 50% of their pain had been relieved, and 13 believed that stimulation had little or no effect on their pain.
In terms of sites, they had their greatest success with stimulation of the PAG and PVG.
The patients much preferred the sensation evoked by activation of the PVG, including a sense of diffuse warmth and well-being.
Stimulation of the PAG, on the other hand, tended to elicit a feeling of apprehension, impending doom, oscillopsia, and diplopia, which interfered with the patients' willingness to stimulate on a regular basis.
A few patients, who were able to obtain good to excellent pain relief with stimulation of the PAG, utilized stimulation only when their pain was at its most severe level due to such side effects.
