Movements

Electrical stimulation of the brainstem can manipulate the body at will, arrest the heart and lungs, and cause various bodily functions.
Similar phenomena can be elicited in other brain regions, although not as effectively as in the brainstem.
If abused, this would even make it possible, directly by causing cardiac or respiratory arrest, or indirectly by manipulating the body to cause accidents, to assassinate individuals without leaving any evidence.
Table of ContentsAll_Pages
Circuitry
Brainstem
Neural circuits that regulate movements are distributed throughout the nervous system. (Ruder and Arber 2019)
The motor cortex and basal ganglia are higher-level regions involved in motor planning while the spinal cord is the lower-level region that ultimately executes the body movements.
The brainstem is an important interface located between these two regions and harbors distributed neural circuits important for the regulation of diverse motor behaviors.
It is known that elementary movements can be achieved using only functions at and below the brainstem.
Frogs with no forebrain and an intact brainstem can perform most of the behaviors displayed by normal frogs, including jumping, stepping, and swimming. (Roh et al. 2011)
Infants with no forebrain and an intact brainstem (hydranencephaly) cry, smile, suckle, and move their eyes, face, arms, and legs just like normal babies. (Saper et al. 2013)
Motor Modules
It is considered that the vertebrate motor system can produce diverse movements by combining a small number of motor modules. (Bizzi et al. 2008)
A motor module, also called a muscle synergy, is a functional unit in the spinal cord that activates a specific muscle group in a specific pattern to produce a specific motor output.
For example, a defensive kick of the frog is composed of three motor modules, and by changing the amplitude and delay time assigned to each module, a variety of kicking patterns can be generated. (d’Avella et al. 2003)
Without the brainstem, these motor modules will not function, and the brainstem is thought to be the region that regulates discrete motor modules. (Roh et al. 2011, Ruder and Arber 2019)
In fact, electrical stimulation of the brainstem induces a variety of elementary movements, such as flexion and extension of the limbs, opening and closing of the hands, and opening and closing of the mouth.
One experiment applying this showed that, by connecting a computer to the brainstem of monkeys and programming elementary movements to be induced in sequential and parallel, they can manipulate the body of the monkeys at will.
Surprisingly, this experiment was conducted over 50 years ago, in the 1960s.
Locomotion and Bio-Robots
Locomotion
Locomotion, such as walking and swimming, is a fundamental motor behavior common to most animals and humans, and is in large part accomplished by activity of the executive circuits in the spinal cord. (Leiras et al. 2022)
Immediate central control over these spinal circuits is located in the brainstem, which exerts control over the initiation, speed, termination, and direction of locomotion.
It has been shown that electrical stimulation of the brainstem can induce locomotion in animals, including walking, running, swimming, and turning.
Bio-Robots
The merging of the biological and the artificial represents a new trend in robotics, and merged hybrid animals are referred to as bio-robots, animal cyborgs, or animal-robots. (Romano et al. 2018)
One of the approaches to realizing bio-robots is to control animal locomotion by electrical brain stimulation.
This makes it possible to remotely control animals wirelessly like radio-controlled cars, or connect them to the computer to run autonomously along a designated route.
Autonomic Nervous System
The motor arm of the autonomic nervous system regulates physiology within a variety of systems including respiratory, cardiac, vasomotor, digestive, and endocrine. (Beissner et al. 2013)
Some of the most important control centers for autonomic functions are located in the brainstem.
In addition to the brainstem, the hypothalamus, insular cortex, cingulate cortex, and amygdala form the core of the central autonomic network.
The autonomic nervous system controls the heart, and its direct input comes from the brainstem. (Silvani et al. 2016)
The brainstem also harbors the respiratory center, and this circuit is essential for automatic and continuous breathing. (Saper et al. 2013, Bianchi and Gestreau 2009)
Electrical stimulation of the brainstem can cause cardiac and respiratory arrest.
Electrical stimulation of the brainstem can also induce the movements of the autonomic nervous system, such as swallowing, sneezing, coughing, vomiting, urination, and defecation.
Body Manipulation
Computer-Based Motor Control
In 1966, Dr. Lawrence Pinneo of the Stanford Research Institute (now SRI International) connected the brainstem of monkeys to a computer and successfully made the monkeys perform a series of programmed movements. (PINNEO 1966, Pinneo et al. 1972)
The experiment was funded by the Office of Naval Research, and from its content it can be read as having military applications in mind.
In this experiment, the brainstem and cerebellum of the three rhesus monkeys were connected to a computer via cables, and the computer was used to manipulate these motor neurons in an attempt to make the monkeys perform complex movements.
The doctor first analyzed goal-directed behavior of monkeys, such as eating, grooming, locomotion, play, and aggressive behavior, and broke them down into their components, elementary movements.
Sequential and parallel elicitation of these elementary movements would, the doctor believed, produce coordinated limb movements, and thus make the monkey perform a variety of complex movements.
Next, extensive functional brain mapping was conducted to determine which sites in the brainstem and cerebellum needed stimulating to produce various elementary movements.
Electrodes were placed into the nuclei of the cerebellum and the vestibular nuclei of the brainstem, as well as the reticular formation and many other brainstem structures, and over 200 locations were found in which elementary movements could be produced by electrical stimulation.
These elementary movements included flexion and extension of all four limbs at the wrist, elbow, shoulder, ankle, knee, or hip; clenching and spreading of the fingers and fine digital movements; opening and closing of the mouth; movements of the tongue in and out; curling or sideways movement of the tail; movements of the eyes, singly and together, and dilatation of the pupils; and many autonomic responses such as modification of heart or respiration rate.

In addition, it was found that the movements obtained from the brainstem and cerebellum were much more precise than can be elicited by stimulation of the motor cortex.
Up to as many as 60 electrodes were then chronically implanted in the brains of the monkeys and connected to a computer via cables.

The computer could control up to 10 electrodes simultaneously.
Finally, the combination of the elementary movements was written as a program, which was then executed by a computer.
Just as he expected, Dr. Pinneo was able to make the monkeys perform a variety of goal-directed behavior.
These included, for example, movements to reach out the limb to grasp an object such as food and bring it rapidly and smoothly to the mouth; to extend the arm outward and upward in climbing; and to extend the arm backward over the back of the body and move it back and forth as in scratching at the base of the tail.
The elementary movements in the three monkeys were very similar when stimulated in homologous sites, and a given sequence of stimulation produced essentially the same sequence of behavior in each monkey. Abnormally appearing temporal sequences, or "slow motion" behavior, could be obtained as well.
This sequence could be repeated as often as desired, and the movements could not be discriminated by observers from those which occurred when the monkey voluntarily performed the same act.
It appeared that an almost endless repertoire of sequential movements could be elicited by simply changing the program and stimulation duration.
In all cases, the electrical stimulation overrode any voluntary movement of the monkey, and essentially the same sequential movement could be elicited from whatever the position of the monkey, and whether the monkey was asleep, awake, or under anesthesia.

Bio-Robots (Locomotion Control)
There are sites in the brainstem that can induce locomotion by electrical stimulation, such as moving forward, turning left, or turning right.
This makes it possible to remotely control animals wirelessly like radio-controlled cars, or connect them to the computer to run autonomously along a designated route.
In other words, this makes it possible to turn animals into bio-robots.
Forward Movements
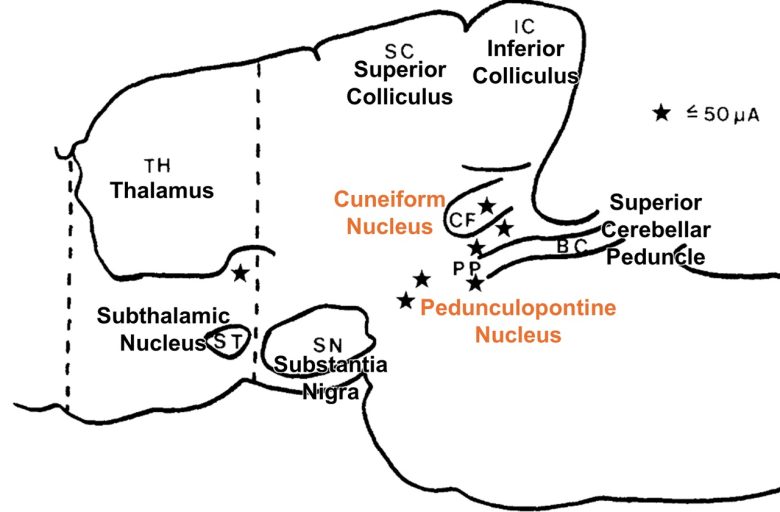
Russian researchers found that electrical stimulation of the cuneiform nucleus in the midbrain in cats can induce walking and running. (Shik et al. 1966, Fridman 1969)
At a fixed intensity of stimulation the cat ran at constant speed, making quite coordinated movements.
By regulating the strength of stimulation, the running speed could be changed, causing the cats to change their gait from walking to running.
These basic characteristics of walking and running were similar to those seen normally.
An experiment with monkeys has also shown that electrical stimulation of the cuneiform nucleus can induce walking and running. (Eidelberg 1981)
Electrical stimulation of the cuneiform nucleus in the crab-eating macaques induced rhythmic alternating movements of the limbs similar to walking.
With the lower stimulation, the hindlimb on the opposite side began stepping 2-5 seconds after the onset of the stimulation and continued to step for 2-3 seconds after the end of the stimulation.
Stronger stimulation usually recruited the other hindlimb first and then the forelimbs with a rhythmicity resembling that of a slow walk.
Still stronger stimulation caused the stepping rate to increase and then shift to a gallop pattern.
Researchers at the University of Arkansas conducted experiments with rats to broadly explore brain regions that induce walking. (Skinner and Garcia-Rill 1984)
They found that walking can be induced not only from the cuneiform nucleus but also from the pedunculopontine nucleus.
Other than that, there was a site just below the thalamus that could induce walking.
In this experiment as well, they were able to change the walking speed by varying stimulation intensity.
Weak stimulation induced walking, medium stimulation induced a trot, and strong stimulation induced a gallop.
Another study has shown that stimulation frequency also changes walking speed. (Caggiano et al. 2018)
Increasing the stimulation frequencies from threshold frequency of 5 Hz to optimal frequency of 50 Hz progressively increased the speed of locomotion.
This region, consisting of the cuneiform nucleus and pedunculopontine nucleus, is called the "mesencephalic (midbrain) locomotor region," and has been found in diverse vertebrate species, including the lamprey, skate, rodent, pig, monkey, and human. (Noga and Whelan 2022)
Turning Movements
A study at Hiroshima University in Japan showed that electrical stimulation of the medial longitudinal fasciculus in goldfish can induce not only forward movements but also turning movements. (Kobayashi et al. 2009)
In the experiment, a micro wireless stimulation unit for fish was developed. The unit was fitted with a float and attached to the goldfish.
Electrical stimulation of the sites on the midline in the medial longitudinal fasciculus in the goldfish induced forward movements.
On the other hand, electrical stimulation of the sites off the midline induced turning movements toward the side stimulated.
By sending these instructions from a radio transmitter to the stimulation unit, they were able to remotely control the goldfish, like a radio-controlled toy.
A study at Yanshan University in China showed that electrical stimulation of the cerebellum in carp can induce four types of locomotion: turning right, turning left, moving forward, and moving backward. (Peng et al. 2011)
Stimulation of the upper site on the midline in the cerebellum caused the carp to move forward, while stimulation of the lower site induced them to move backward.
Stimulation of the sites on the left of the midline in the cerebellum caused the carp to turn right, while stimulation of the sites on the right induced them to turn left.

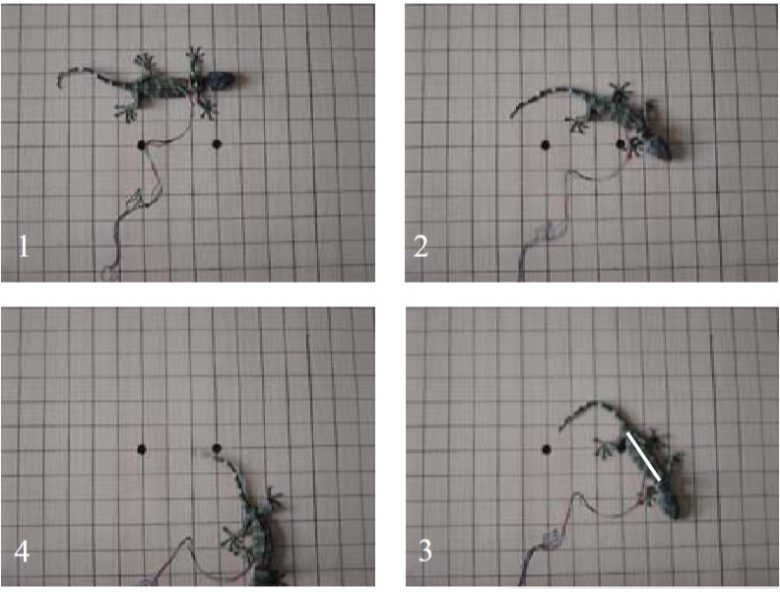
An experiment with geckos showed that forward movements and turning movements can be induced from large regions of the midbrain tegmentum. (Wenbo et al. 2009)
Electrical stimulation of the sites near the midline of the midbrain tegmentum induced forward movements, while stimulation of the sites off the midline induced turning movements.
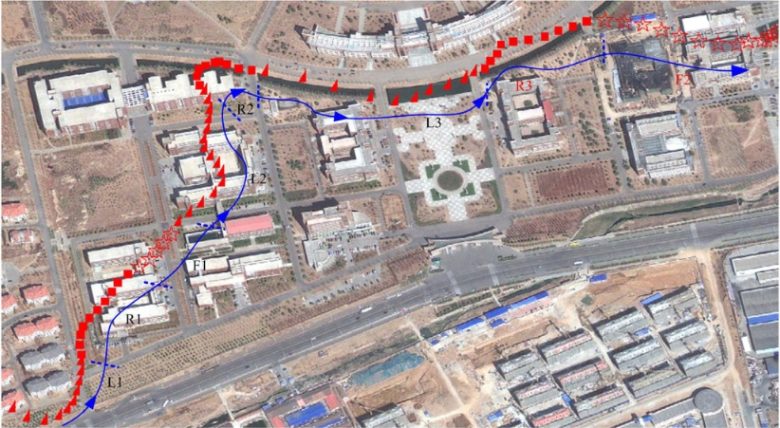
An experiment with pigeons has shown that flight movements can be controlled by electrical brain stimulation. (Yang et al. 2017)
Electrodes were implanted in a sensory relay nucleus of the thalamus, and electrical stimulation of this nucleus acted as a left and right turning command.
Directional instructions were given by a small computer attached to the pigeons.
When given a series of turning commands in flight, the pigeons were observed to change course accordingly.
Bio-Robots
As a simple example of making animals bio-robots, researchers at the University of Tokyo succeeded in keeping a cockroach walking autonomously along a black line using antenna stimulation. (Holzer and Shimoyama)
When a cockroach is stimulated by the antenna, it will flee to the opposite side.
In the experiment, a small control device was developed that applies electrical stimulation to the antennae when the insect strays from the black line.
A cockroach fitted with this device received antenna stimulation every time it strayed from the black line, causing it to turn back onto the line.
Thereby, they were able to make the cockroach walk autonomously along the black line.
The SUNY Downstate Medical Center developed a technology that allows them to remotely control rats like radio-controlled cars. (Talwar et al. 2002)
Electrodes that deliver electrical stimulation via wireless were implanted into the medial forebrain bundle and the areas in the sensory cortex that represent the left and right whiskers.
Rats moved forward when stimulated in the medial forebrain bundle, and turned in that direction when stimulated in the left or right whisker areas of the sensory cortex.
This enabled them to remotely control the rats from a distance of 547 yd (500 m).
Stimulation of the medial forebrain bundle motivated the rats not only to move forward but also to climb up and down steps that were faced.
Thus, stimulation of the medial forebrain bundle was sufficient to steer the rats through a wide variety of complex, new, and changing terrains.
The rats were easily guided through pipes and across elevated runways and ledges, and could be instructed to climb or jump from any surface that offered sufficient purchase such as trees.
They were also able to guide the rats in systematically exploring large, collapsed piles of concrete rubble, and to direct them through environments that they would normally avoid, such as brightly lit, open arenas.
The researchers named the rats "robo-rats" and expressed their enthusiasm for applying the technology to make animals bio-robots.

Inspired by the robo-rats, a research group at Zhejiang University, China, developed a system that connects the brains of rats to the computer to run autonomously along a designated route. (Wang et al. 2015)
First, the rats were fitted with a miniature camera and a wireless brain stimulation device.
The camera captured the scene in front of the rats and transferred it wirelessly to the computer.
Next, the computer then detected the indication signs and sent one of three signals to the stimulator wirelessly: turn left, turn right, or go forward, depending on the indication signs.
As in the SUNY study, moving forward was achieved by electrical stimulation of the medial forebrain bundle, and turning left or right was achieved by that of the whisker areas in the sensory cortex.
They then created an urban model with buildings, trees, roads, and junctions, with indication signs of left and right arrows and of a human face (moving forward).
When the rats were placed in this model, it was confirmed that the rats actually moved in the urban model following the signs that had been placed.

The research group took this a step further and, as an example of the integration of machine intelligence and biological intelligence, showed that a computer can assist rats to escape from a maze and solve the maze faster. (Yu et al. 2016)
The rats received computer assistance, i.e., electrical brain stimulation, with the following rules.
- If there was a path to the unique road, the computer would find the shortest path, and then left and right commands would be sent to navigate the rat to the unique road.
- If the rat was going to enter a dead cell, a left or right command would be sent to prevent such a move.
- If the rat was in a loop, the computer would find the shortest path to the current destination, and then left and right commands would be sent to navigate the rat to follow the path.
The rats solved 14 different mazes, and steps, coverage rates, and time spent were evaluated.
They found that the computer-assisted robo-rats outperformed the normal rats in maze-solving.
Dr. Hess's Animal Experiments
Dr. Walter Hess of the University of Zurich in Switzerland discovered that electrical stimulation of the diencephalon (thalamus and hypothalamus) in cats can induce a variety of movements. (Hess and Brügger 1943, Hess 1958)
Head Movements
Stimulation of the region lateral to the posterior commissure immediately caused the cat to lower its head, and as stimulation continued, the cat repeatedly tapped its nose against the table.
When this region was electrically coagulated, the cat ended up with its head left held up.
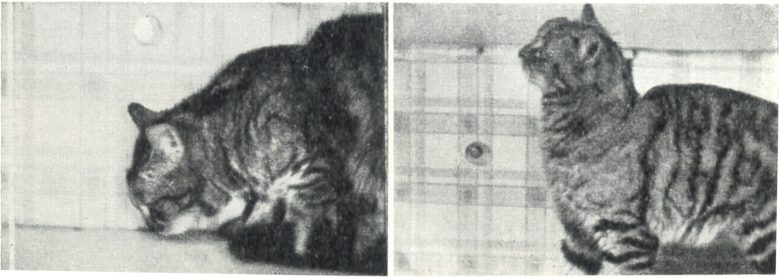
Electrical stimulation of the medial part of the posterior hypothalamus caused the cat to elevate its head and, in some cases, the entire front part of the body.
The response began with the start of stimulation and led, in a few sudden movements, to a position with the head held high.
When the cat was stimulated while lying down, it raised itself forward. With increasing intensity of stimulation, this effect could develop further so that the front part of the body was elevated from the floor until the cat fell over backward.
In falling, the cat quickly rotated on its longitudinal axis to avoid landing on its back.

Rotational Movements
Rotational responses appeared very often, which were elicited from the region from the thalamus to the subthalamus.
The rotatory movements elicited in the more anterior and more dorsal region were mainly related to the head, and were sometimes limited to it.


Stimulation of the more posterior and more ventral region, however, elicited the effects not only on the head but also on the anterior part of the body and eventually on the entire body, so that the rolling movement easily developed.
These effects were more pronounced with increasing stimulation intensity.
As soon as the rotation proceeded so far that the cat was on its back, it drew itself in one jerk in the direction of the artificially induced movement so rapidly that it immediately sprang to its feet again.

Limb Movements
Stimulation of the ventral part of the thalamus produced three types of forelimb movements in cats: drawing up, adduction (moving inward), and abduction (moving outward).
The phenomenon was also observed that the forelimb was moving up and down in sync with the stimulation frequency.

Facial Movements
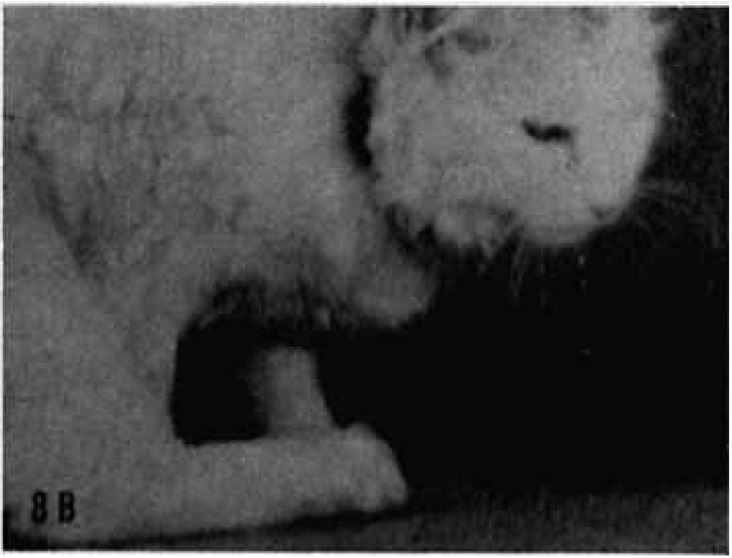
Motor responses in the face were observed very frequently and obtained easily in the ear muscles, eyelids, and whiskers.
By far the most common response was a turning backward of the whiskers on the opposite side.
If the stimulation effect was strong, the movement extended, for example, from the whiskers to the upper lip, which was retracted to the opposite side. Sometimes the cheek on the opposite side also participated and was elevated.
Often, with slightly stronger stimulation, the whiskers on the same side also moved, but forward instead of backward.
Similar behavior was observed in the ears, and the most common reaction was a turning of the ear on the opposite side backward, with a turning of the ear on the same side forward.
In the eyelids, the movements occurred on the opposite side with few exceptions.
Sometimes the movement spread over to the same side, where, however, it was always considerably weaker. The eyelids on the opposite side were entirely shut, while on the same side the eyelids were only slightly closed and showed a slight blinking in sync with the stimulation rhythm.

The sites that elicited facial responses lay in the higher levels of the diencephalic motor area, namely, in the zones passing through the thalamic radiations.
Delgado's Animal Experiments
Yale University neurophysiologist Jose Delgado conducted many experiments that induced movements by electrical brain stimulation, primarily on monkeys and cats, but also on humans. (Delgado 1964, Delgado 1970)
Brain stimulation of different sites elicited most of the simple movements observed in spontaneous behavior.
This included frowning, opening and closing the eyes, opening and closing the mouth, movements of the tongue, chewing, contraction of the face, movements of the ears, turns, twists, flexions, and extensions of the head and body, and movements of the arms, legs, and fingers.
Abnormal responses, disorganized movements, loss of equilibrium, and epilepsy-like convulsions were also produced, depending on the cerebral regions and stimulation parameters.
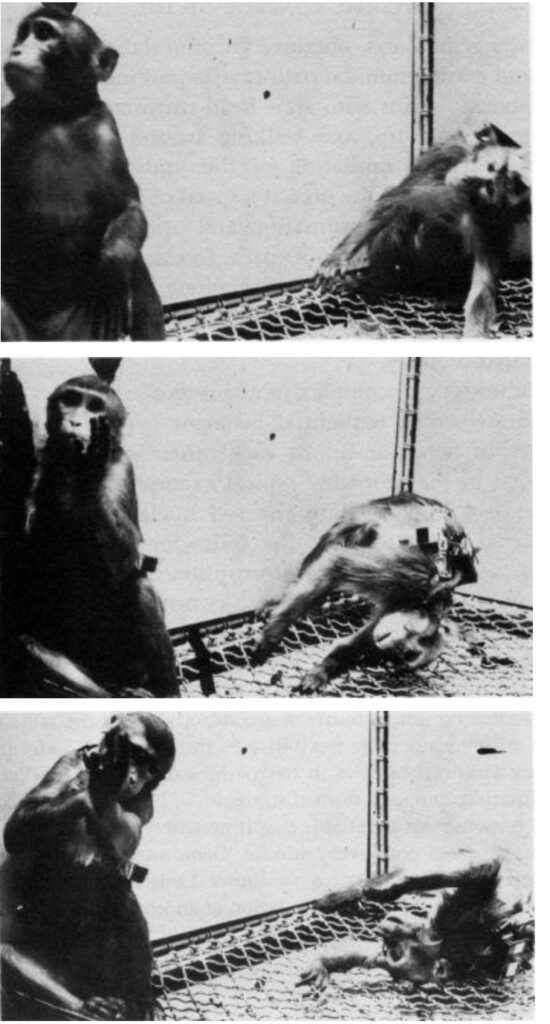
For example, electrical stimulation of the tectum in a monkey produced complete loss of equilibrium, and the monkey collapsed, rotating clockwise on the longitudinal axis.
The diameter of the pupil could be adjusted at will from maximum constriction to maximum dilatation, as if it were a camera, simply by turning the intensity knob of the stimulator connected with the hypothalamus.

Surface Brain Stimulation
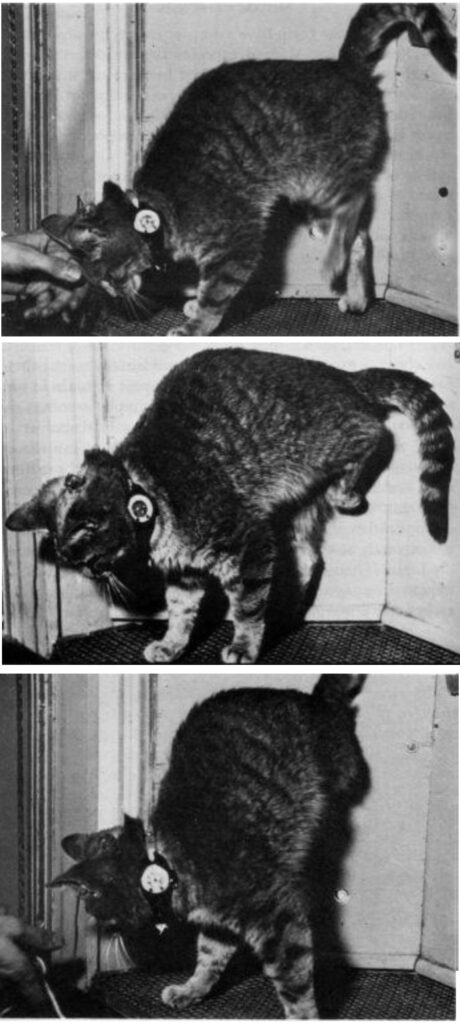
Electrical stimulation of the right motor cortex of cats induced flexion of the left hindlimb, with the magnitude of the movement proportional to the intensity applied.
For example, when the cat was standing on all fours, the intensity of 1.2 mA evoked flexion of the hind leg barely off the ground. At 1.5 mA the leg rose about 4 cm (1.6 in), and at 1.8 mA leg flexion was complete.
In monkeys, electrical stimulation of the motor cortex evoked movements on the opposite side to the stimulation, just as in cats.
In a human subject, electrical stimulation of the left parietal cortex evoked a flexion of the right hand starting with contraction of the first two fingers and continuing with flexion of the other fingers.
The closed fist was then maintained for the rest of the 5-second stimulation.
This effect was not unpleasant or disturbing, and it developed without interrupting ongoing behavior or spontaneous conversation.
When the subject was warned of the incoming stimulation and was asked to try to keep his fingers extended, he could not prevent the evoked movement and commented, “I guess, Doctor, that your electricity is stronger than my will.”
If this stimulation was applied while the subject was voluntarily using his hand, for instance to turn the pages of a magazine, this action was not blocked but the induced hand flexion distorted voluntary performance and resulted in crumpling and tearing of pages.
Electrical stimulation of the motor cortex did not induce precise or skillful movements, and in all cases the evoked responses were clumsy and abnormal.
Deep Brain Stimulation
Movements evoked by stimulation of regions deep within the brain, such as the limbic system and the thalamus, were usually more complex than those produced by motor cortex stimulation, and appeared purposeful.
For example, in a human subject, electrical stimulation of the rostral part of the internal capsule produced head turning and slow displacement of the body to either side with a well-oriented and apparently normal sequence, as if the subject were looking for something.
This stimulation was repeated six times on two different days with comparable results.
The subject considered the evoked activity spontaneous and always offered a reasonable explanation for it.
When asked, “What are you doing?” the answers were “I am looking for my slippers,” “I heard a noise,” “I am restless,” and “I was looking under the bed.”
Reproducibility of Stimulation
When stimulation was applied to one point of the brain, a similar effect could be consistently elicited after intervals of hours, months, or years. (Delgado 1959)
Reproducible effects included bodily responses such as turning the head; autonomic responses such as pupillary constriction and dilation; and behavioral effects such as fright, escape, and vocalization.
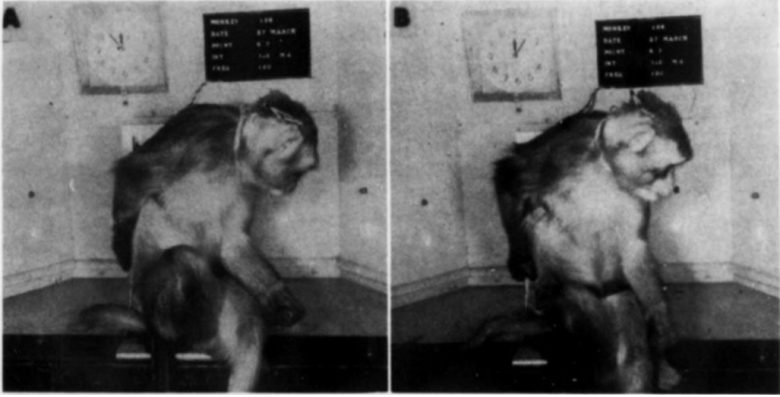
Electrical stimulation of the left rhinal sulcus in a monkey evoked head turning to the right, opening the mouth without showing the teeth, and scratching of the left face with the left hand.
When the same point was stimulated 60 times during 1 hour, the same response was repeated every minute.

If the monkey was eating or walking at the onset of the stimulation, the behavior was interrupted, and the effects of opening the mouth, turning the head, and scratching appeared, and then the monkey resumed the original behavior as soon as the stimulation ceased.
Electrical stimulation of a point in the left side between the substantia nigra and nucleus of the oculomotor nerve evoked contraction of the left orbicularis muscle and turning of the left eye to the right.
Over a period of 4 years and 2 months this point was stimulated more than 100 times, and the evoked effect remained constant.
Stimulation of a point in the right putamen near the internal capsule evoked extension of the left leg, flexion of the left hand and arm, turning and some flexion of both head and body to the left, and turning of both eyes to the left.
This movement was also repeatable over a long period of time.
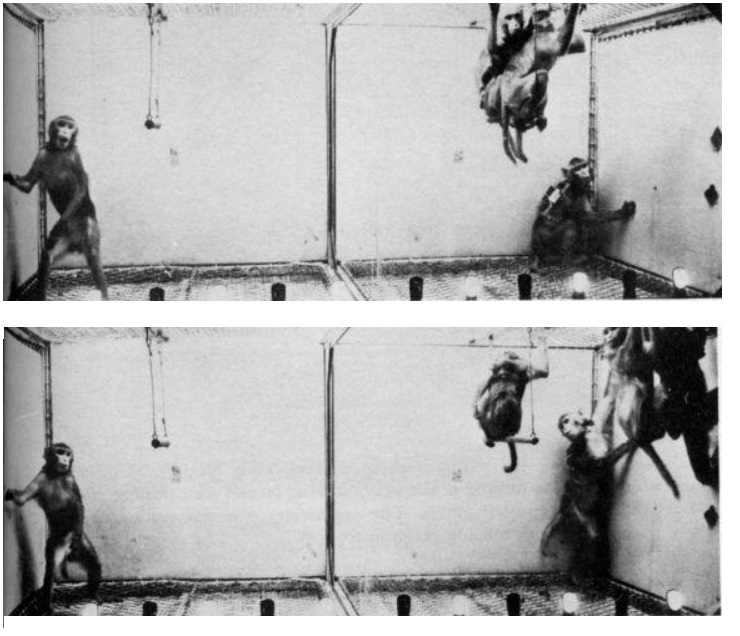
Induction of Running
Electrical stimulation of the fimbria in monkeys induced an immediate and violent running response, which appeared without exception each time the stimulation was applied. (Delgado 1967)
The monkeys ran from side to side in the cage at great speed during all the stimulation periods, climbing halfway up the side of the cage to spring back with great energy.
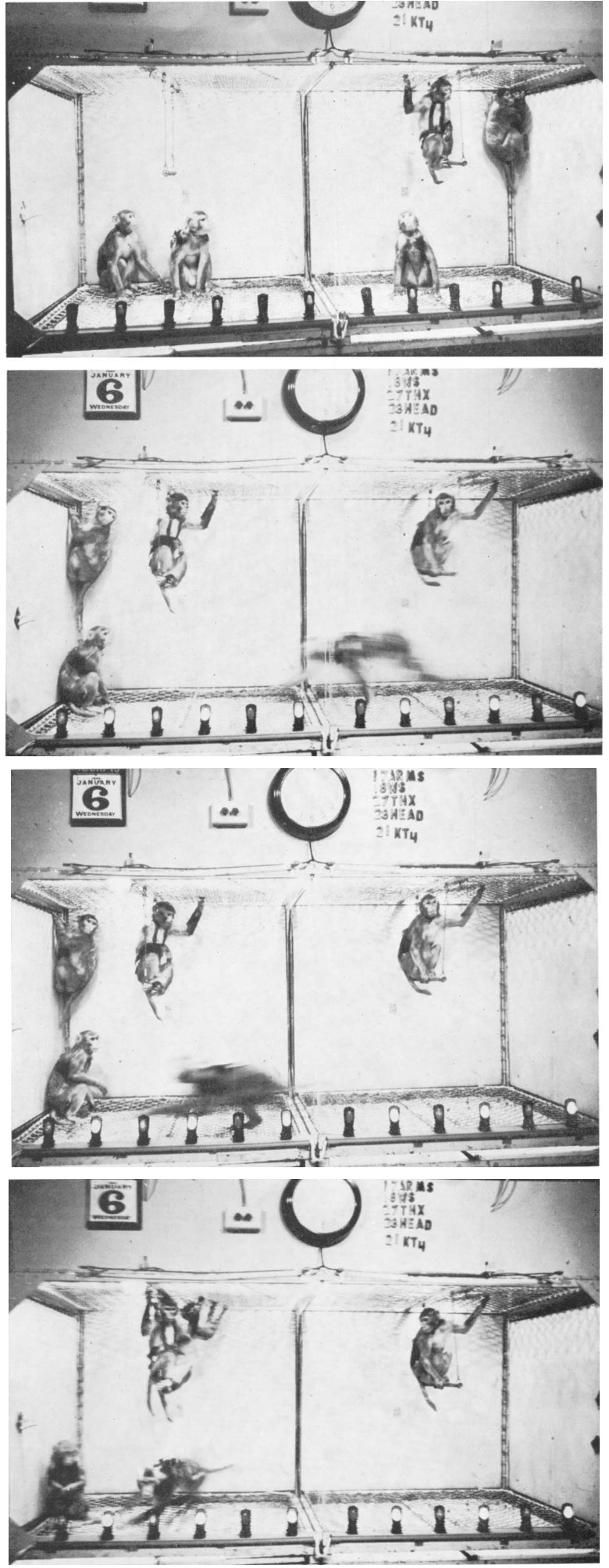
Coordination of movements was excellent, and orientation was well preserved.
They were able to avoid clashes with the other colony monkeys, and when occasionally one of them was run over, there was no fight or aggression.
After several trials, the whole floor of the cage was free for the racing monkey, while the others sat on the swings or hung in the corners.
As soon as stimulation was over, the stimulated monkey sat down.
If food was offered during stimulation, the monkey paid no attention to it and continued running around. Even threatening was ineffective.
Delgado arranged so that when the lever in the cage was pressed, electrical stimulation of the fimbria was delivered to the experimental monkey. (Delgado 1970)
One monkey then learned to press the lever, and electrical brain stimulation to the experimental monkey began.
As this stimulation was repeated, the experimental monkey became conditioned to the lever pressing of the manipulating monkey.
That is, the experimental monkey became such that, as soon as the manipulating monkey approached the lever, he showed restlessness and stood in the cage corner, ready to start running.
Respiratory Arrest/Apnea
Respiratory Arrest
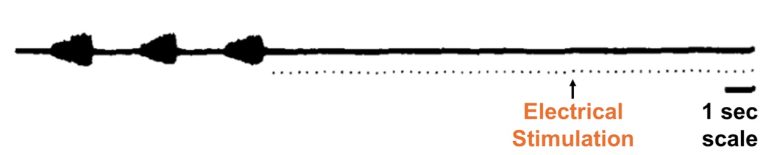
Electrical stimulation of the brainstem can cause permanent cessation of breathing, or respiratory arrest.
Brainstem
A study at Paul Cézanne University in France (now Aix-Marseille University) discovered that electrical stimulation of the brainstem in cats causes permanent respiratory arrest. (Bassal and Bianchi 1982)
In the experiment, they explored various brain regions in the cats that affect breathing.
The onset of phrenic nerve activity was obtained by electrical stimulation of the primary sensory cortex, the periaqueductal gray, the reticular formation, the parabrachial nucleus, the vestibular nucleus, and the fastigial nucleus.
In particular, in brainstem structures such as the periaqueductal gray and reticular formation, respiration was entrained to the stimulation frequency when stimulation was repeated at frequencies between 1 and 5 Hz.


The termination of phrenic nerve activity was obtained by electrical stimulation of the motor and visceral cortices, the internal capsule, the pyramidal tract, the red nucleus, the rubrospinal tract and the motor nucleus of the trigeminal nerve.
In particular, in brainstem structures such as the pyramidal tract and rubrospinal tract, respiration weakened or even permanently arrested when stimulation was repeated at frequencies between 1 and 10 Hz.

Apnea
Other brain regions have also been shown, without going as far as respiratory arrest, to induce temporary cessation of breathing, or apnea.
Orbitofrontal Cortex
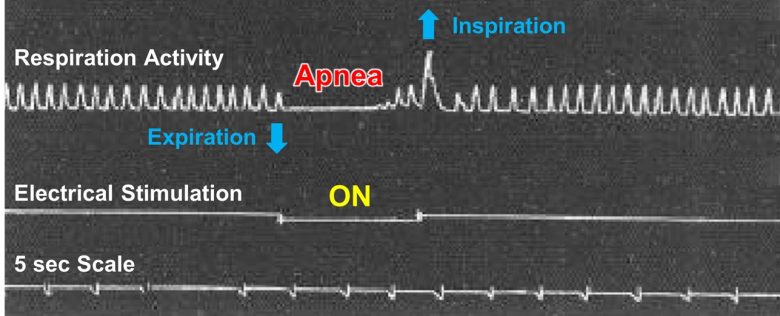
William Sweet of Massachusetts General Hospital in Boston found that electrical stimulation of the orbitofrontal cortex in cats and monkeys can induce apnea. (Bailey and Sweet 1940)
Weak electrical stimulation depressed breathing, while strong stimulation ceased breathing.
Less than 1 second after the stimulation onset, the animals ceased breathing in expiration for 5–10 seconds and escaped with slow, shallow breathing.
After the stimulation ended, they took a deep breath, resuming normal breathing rate and amplitude at once.
The responses were essentially the same for the cats and monkeys.
At Boston Psychopathic Hospital, a human experiment was conducted in which psychiatric patients scheduled for lobotomy were given electrical stimulation to induce apnea. (CHAPMAN 1949)
Seven patients had schizophrenia, and three had mixed psychosis.
Electrical stimulation of the orbitofrontal cortex caused depression of or cessation of breathing in seven patients.
Respiratory effects occurred within 1-2 seconds of electrical stimulation.
Recovery of respirations usually occurred from 2 to 4 seconds after the end of the stimulation, although in 2 patients apnea continued as long as 40 seconds after each of three stimulations.
These responses were able to be reproduced by stimulating the same area.
Anterior Cingulate Cortex
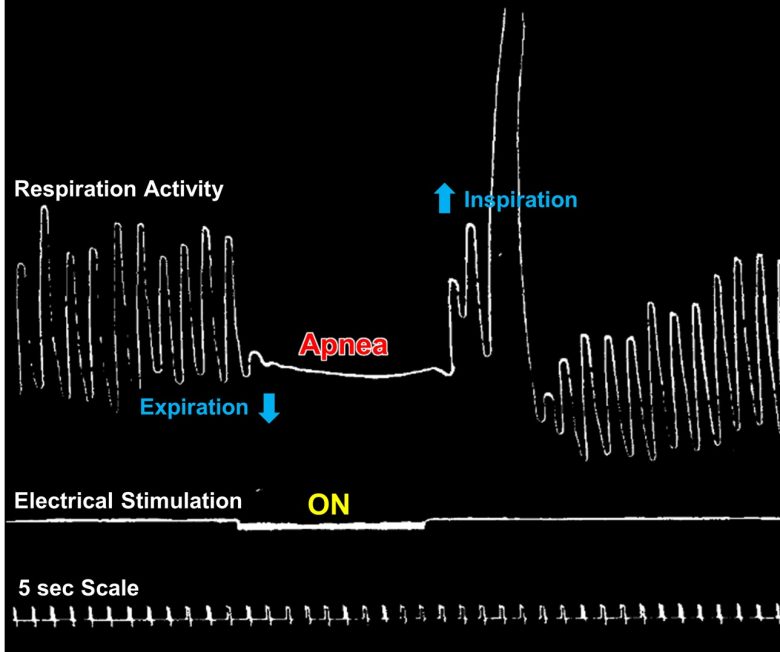
Electrical stimulation of the anterior cingulate cortex has also been shown to produce apnea. (Smith 1945)
Electrical stimulation of the anterior cingulate cortex in the rhesus monkeys caused cessation of breathing after a short delay.
The respiratory movements were held in abeyance during the entire period of stimulation, provided it was no longer than several seconds in duration.
The cessation occurred in expiration, and could continue for 1–2 seconds after stimulation ended.
Respirations then began again, at first shallow, but with gradually increasing depth so as to reach the pre-stimulatory level within a few seconds.
In many instances the apnea was followed by deep breathing, after which respirations quickly returned to normal.
At Columbia University, a human experiment was conducted to investigate the effects of electrical stimulation of the anterior cingulate cortex on the autonomic nervous system. (Pool and Ransohoff 1949)
The subjects were 11 schizophrenic patients undergoing lobotomy.
Electrical stimulation of the anterior cingulate cortex caused cessation of breathing.
The respiratory rate first fell, leading to a period of complete apnea lasting for about 30 seconds after the termination of stimulation.
Amygdala
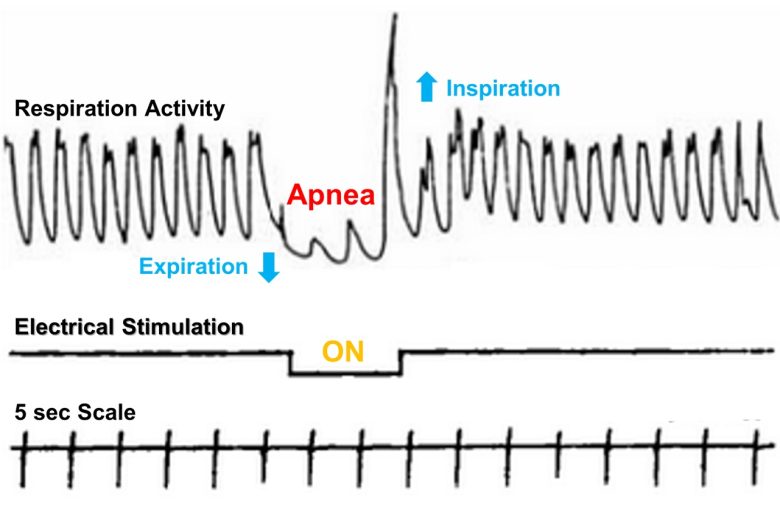
Dr. Birger Kaada of the University of Oslo showed that electrical stimulation of the amygdala can also produce apnea. (Kaada et al. 1954)
Electrical stimulation of the anterior medial part of the amygdala in cats produced an immediate inhibition of respiratory movements. The chest usually assumed a position in expiration during the period of apnea.
The respiratory movements could not ordinarily be held in abeyance for more than 40-60 seconds despite continuous stimulation, after which period escape occurred.
In a few animals, however, respiration ceased for a considerable period after interruption of the stimulation, which necessitated artificial respiration in order to stimulate the animal to spontaneous respiration.
Other Regions
Dr. Kaada went on to extensively search for regions in the cerebral cortex in monkeys that cause respiratory responses. (Kaada et al. 1949)
The doctor found that electrical stimulation of wide regions of the brain can induce depression of or cessation of breathing.
The sites that induced these responses were in a continuous stretch extending through the temporal pole, anterior insular cortex, posterior orbitofrontal cortex, anterior cingulate cortex, and septal area.
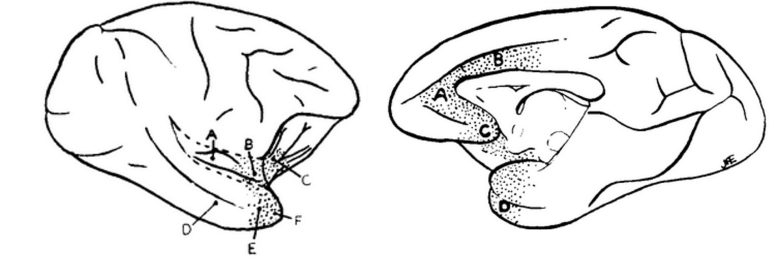
Left: Medial brain regions. Right: Lateral brain regions.
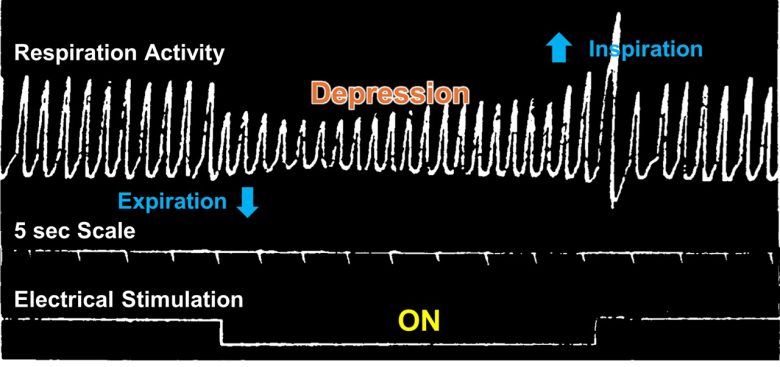
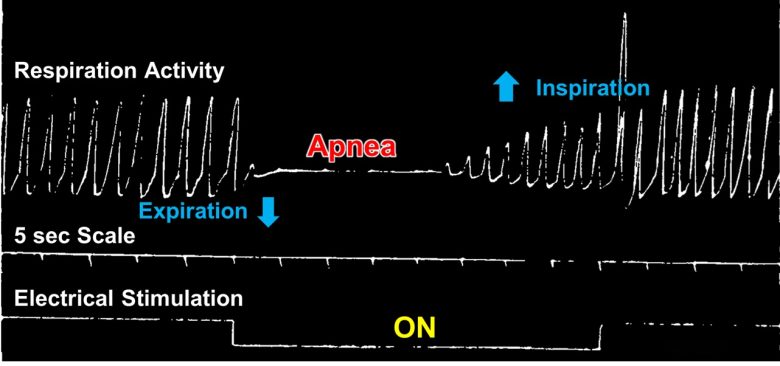
Weak electrical stimulation depressed breathing, while strong stimulation ceased breathing.
When complete apnea occurred, the chest assumed a position in expiration.
The apnea was not maintained for more than 25-30 seconds, after which period the respiratory movements reestablished themselves despite continued stimulation.
Cardiac Arrest/Arrhythmias
Cardiac Arrest
Electrical stimulation of the brainstem can cause cardiac arrest.
It has also been shown that complex patterns of electrical stimulation of the insular cortex can cause cardiac arrest.
Brainstem
A McGill University study discovered that electrical stimulation of the brainstem in rats can produce cardiac arrest. (Malmo and Mundl 1983)
The experiment was conducted to investigate the side effects of electrical stimulation of the midbrain regions, which had been shown to have analgesic effects in humans.
They found that electrical stimulation of the superior cerebellar peduncle in the midbrain in rats caused cardiac arrest.
This was able to be reproduced by electrical stimulation of the same point.
Oxygen was immediately administered intranasally, and subsequent recovery was characterized by a steep rise in mean arterial pressure.
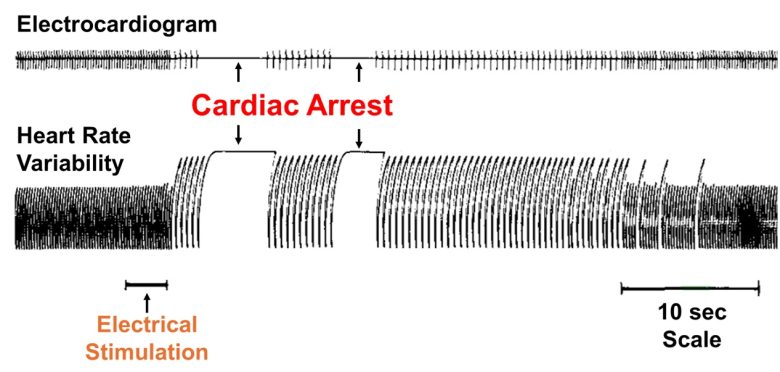
Electrical stimulation of other midbrain regions was shown to cause increases and decreases in blood pressure and changes in breathing.
Insular Cortex
It has been implicated that the cerebral cortex is involved in arrhythmias and cardiac arrest. (Oppenheimer and Cechetto 1990)
Dr. Stephen Oppenheimer of Johns Hopkins University turned his attention to the insular cortex, implicated in autonomic control, and conducted experiments of electrical stimulation of this region.
The doctor found that electrical stimulation of the insular cortex in sync with the heartbeat of the rats produced tachycardia and bradycardia.
Tachycardia was induced from the rostral part of the posterior insular cortex, began an average of 8 seconds after stimulation, and produced an increase in heart rate of an average of 15 beats per minute.
Bradycardia was induced from the caudal part of the posterior insular cortex, began an average of 9 seconds after stimulation, and produced a decrease in heart rate of an average of 16 beats per minute.
The stimulation affected purely heart rate and no other parameter.
The doctor furthered his research and investigated whether electrical stimulation of the insular cortex could induce cardiac arrest from arrhythmias. (Oppenheimer et al. 1991)
Three 5 ms pulses were delivered to the tachycardiac-inducing region of the insular cortex, in sync with the heartbeat of the rats, 100 ms before the T wave of the electrocardiogram, once every two cycles.
This was estimated to produce a burst of sympathetic activity during the period of maximal cardiac electrical instability.
As a result, all seven stimulated rats developed marked electrocardiogram changes and arrhythmias within eight hours.
Over several hours, the electrocardiogram abnormality progressively developed, and subsequently, premature ventricular contractions appeared and complete heart block occurred.
The cardiac rhythm became increasingly erratic and eventually a marked bradycardia was observed.
Cardiac arrest finally occurred, leading to the death of the rats.
Arrhythmias
Other brain regions have also been shown, without going as far as cardiac arrest, to induce arrhythmias like premature contractions.
Hypothalamus
A study at the University of Amsterdam found that electrical stimulation of the hypothalamus in cats can induce arrhythmias. (Korteweg et al. 1957)
Electrical stimulation of the anterior part of the hypothalamus produced bradycardia, an immediate reduction in heart rate from 190 beats per minute to 100 beats per minute.
Electrical stimulation of the posterior part of the hypothalamus produced hypertension, a considerable increase in blood pressure from approximately 125 mmHg to 200 mmHg. Also, premature contractions frequently occurred after stimulation ended.
A study at the University of California also showed that electrical stimulation of the hypothalamus in cats can induce arrhythmias. (Weinberg and Fuster 1960)
Electrical stimulation of primarily the posterior and lateral regions of the hypothalamus produced a variety of arrhythmia symptoms.
These included atrioventricular dissociation, premature contractions, tachycardia, and WPW syndrome.
A McGill University study also showed that electrical stimulation of the hypothalamus in cats can induce arrhythmias. (Melville et al. 1963)
Stimulation of the anterior part of the hypothalamus produced bradycardia, whereas stimulation of the lateral part of the hypothalamus produced tachycardia.
Premature contractions were also observed in both cases.
Anterior Cingulate Cortex
At Columbia University, a human experiment was conducted to investigate the effects of electrical stimulation of the anterior cingulate cortex on the autonomic nervous system. (Pool and Ransohoff 1949)
The subjects were 11 schizophrenic patients undergoing lobotomy.
Electrical stimulation of the anterior cingulate cortex produced bradycardia and tachycardia.
Bradycardia was observed in seven patients, with a maximum pulse fall of 40 beats per minute.
In four patients, these changes outlasted the period of stimulation. The greatest duration of this effect was 10 minutes, and in this case the pulse was grossly irregular with the occurrence of premature contractions.
On the other hand, tachycardia was observed in three patients, with a maximum pulse rise of 40 beats per minute.
Eight patients also experienced an increase in blood pressure, with a maximum rise of 60 mmHg in systolic pressure and 30 mmHg in diastolic pressure.
These changes usually lasted only as long as the stimulation was applied.
Other Brain Regions
Norwegian neurophysiologist Carl Sem-Jacobsen also showed in human experiments on patients with schizophrenia and Parkinson's disease that cardiovascular changes such as arrhythmias occurred by electrical brain stimulation. (Sem-Jacobsen 1968)
Electrical stimulation of the frontal lobe and central part of the brain resulted in cardiovascular changes such as an increase and decrease in pulse rate, an increase and decrease in blood pressure, and premature contractions.
Facial flushing or pallor may also occur, and sometimes, a more complex response was obtained.
Below is a description of arrhythmias that occurred in one patient.
"It is streaming through his chest and body. He likes it very much. He perspires, turns pale while the pulse rate is down to 42, a very brief episode of marked nausea, together with an extremely pleasant feeling. The sensation of nausea like a spice in the dominating feeling of well-being."
Bodily Functions
Electrical stimulation of the brainstem can induce bodily functions such as swallowing, sneezing, coughing, vomiting, urination, and defecation.
The same phenomena have also been observed to occur in the limbic system, including the hypothalamus and amygdala.
Brainstem
Swallowing
Electrical stimulation of the caudal part of the brainstem in cats induced swallowing. (Miller 1972)
Most of the electrode sites evoking swallowing were clustered in the dorsal medullary reticular formation ventral and lateral to the solitary nucleus.
A complete swallowing pattern was elicited at optimal frequencies of 10–30 Hz.
Increasing the intensity of the stimulation caused a “tetanic-like contraction” in the muscle during stimulation, followed by several swallows.
Sneezing
Electrical stimulation of the lower brainstem in cats reproducibly induced sneezing. (Nonaka et al. 1990)
The sneeze-evoking sites were located along the ventral medial part of the spinal trigeminal nucleus and the adjacent pontine-medullary lateral reticular formation.
They tried several stimulation frequencies of 1-50 Hz and a frequency of 10 Hz was found to be most effective.
Coughing
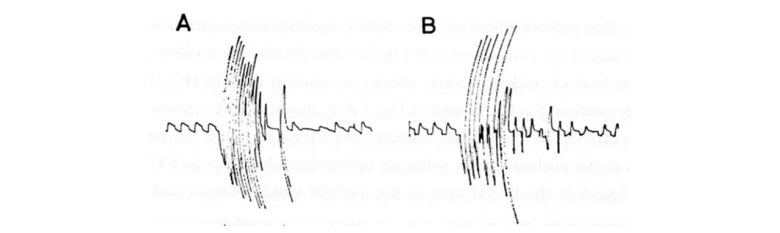
Electrical stimulation of the solitary nucleus in the medulla in cats induced coughing. (MORI and SAKAI 1972)
Brainstem-induced cough was not markedly different from that induced by peripheral nerve stimulation.
Both responses consisted of a series of spasmodic inspiration and explosive expiration of a large amplitude occurring at the rate of one or two per second, as seen in the following tracing of respiration.
Vomiting
Electrical stimulation of a certain region of the medulla in cats induced vomiting. (WANG 1950)
This region corresponded to the dorsolateral border of the lateral reticular formation, including the tractus solitarius and its nucleus.
The optimal stimulation frequency was in the neighborhood of 50 Hz.
When this brain region was destroyed in dogs, the dogs no longer vomited when given vomit-inducing substances.
Urination
Electrical stimulation of a certain region of the pons in cats induced urination. (Holstege et al. 1986)
The electrode sites were located in the medial part of the dorsolateral pontine tegmentum.
Stimulation in this region elicited a prompt decrease in the pelvic floor electromyograph and urethral pressure followed, after a delay of 2 seconds, by an increase in the intravesical pressure, thus simulating normal urination.
However, a complete urination from the stimulation occurred only occasionally.
Defecation
Electrical stimulation of the upper pons in dogs induced defecation. (Okada et al. 1976, Fukuda et al. 1977)
Neurons involved in facilitating defecation were located in the lateral reticular formation medial to the trigeminal motor nucleus in the upper pons.
In contrast, electric coagulation of this region markedly reduced or eliminated the defecation-facilitating activities.
Thalamus and Hypothalamus
Dr. Hess discovered that electrical stimulation of various regions of the diencephalon (thalamus and hypothalamus) in cats can produce various bodily functions. (Hess 1958)
Panting
Tachypnea was obtained from the intermediate and lateral parts of the anterior hypothalamus.
Panting was elicited from much more extensive regions, scattered through the septum, the preoptic area, and the lateral and posterior hypothalamus.
Salivation
The region inducing salivation covered approximately the same region as that inducing panting.
There was an exception to this agreement in that only panting was elicited in the ventral part of the thalamus and in the medial section of the preoptic area.
On the other hand, only salivation was observed after stimulation of points at the anterior margin of the ventral thalamus, especially in the lateral region.
Sneezing
The sites inducing sneezing were rather sparsely distributed in the septum, especially on its border with the adjoining ventricle.
Sneezing could also be elicited in the preoptic area.
Vomiting
The points of stimulation eliciting vomiting were less numerous, and were in the hypothalamus, namely, in the supraoptic area and therefore directly adjacent to the "sneezing point" in the preoptic area, and also in the region around the mammillary body.
There were also two points in the ventral nucleus of the thalamus where vomiting was obtained.
In the hypothalamus, a genuine vomiting occurred in which masses of vomit were ejected from the stomach.
In the thalamus, however, the response had the character of vomiting movements, but no contents were vomited.

Licking
The sites at which licking movements were elicited lay in the medial part of the ventral nucleus of the thalamus.
The licking movements elicited by stimulation of the ventral nucleus made it appear that the cat was licking up or licking at something.
The tongue moved in a regular rhythm; the head was often lowered, while in other cases the cat licked the air.
The licking elicited by stimulation of neighboring points appeared in a different form, such as straight out or obliquely upward.
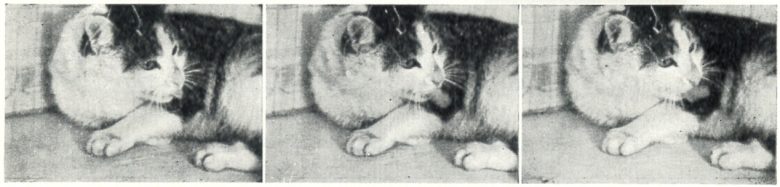
Sniffing
Sniffing was obtained from the stimulation of regions that included the lateral preoptic area and the lateral hypothalamus.
In particular, regions along the medial forebrain bundle produced sniffing at the lowest stimulation intensity.

Urination and Defecation
Urination and defecation were elicited by electrical stimulation of the same topographically indistinguishable region.
The region was clearly circumscribed, beginning in the septal area, the posterior edge of which appeared to be most responsive, crossing the dorsal part of the preoptic area and the supraoptic area, and proceeding into the anterior hypothalamus.
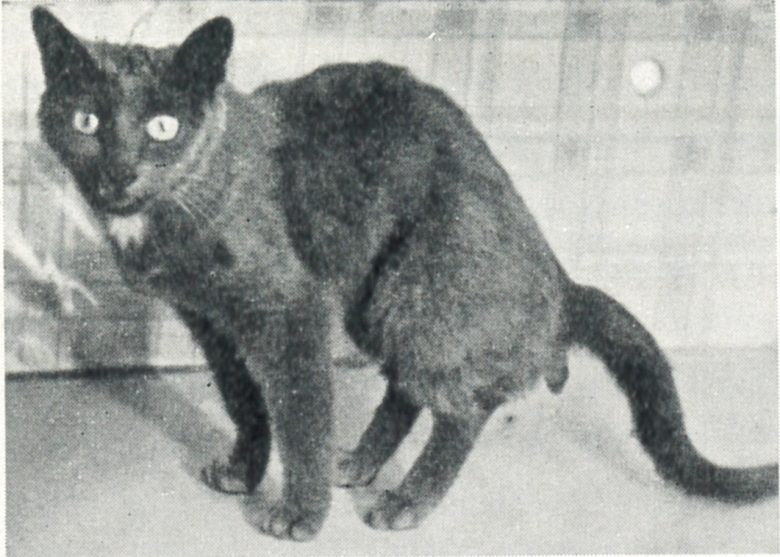
Amygdala
Dr. Kaada showed that electrical stimulation of the amygdala in cats can also produce several bodily functions. (Kaada et al. 1954)
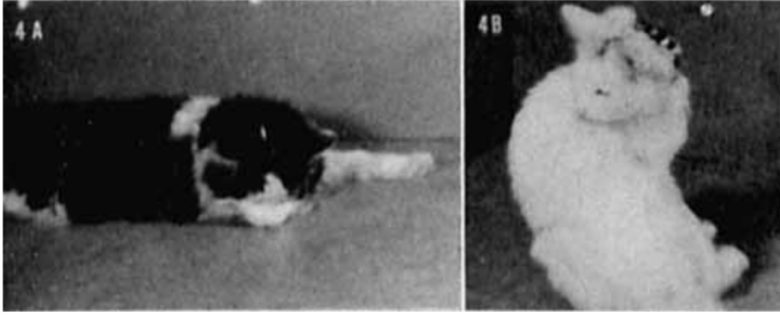
Licking, Sniffing, Chewing
Licking, sniffing, and chewing were most frequently obtained from the medial and anterior parts of the amygdala.
The cat was either licking and sniffing along the floor, or it turned its head slowly with searching movements to the opposite side, licking and sniffing itself.
The licking and sniffing started within a few seconds after the onset of stimulation and continued as long as the stimulus was on, whereas the chewing usually occurred after a delay of 10-15 seconds.
Urination and Defecation
Urination was obtained from the anteromedial nuclei group and the basal nucleus of the amygdala.
The urination usually occurred within a few seconds after the onset of stimulation and could be repeated several times with stimulation of the same points.
Defecation only occurred with stimulation of one point in the anterior part of the amygdala.
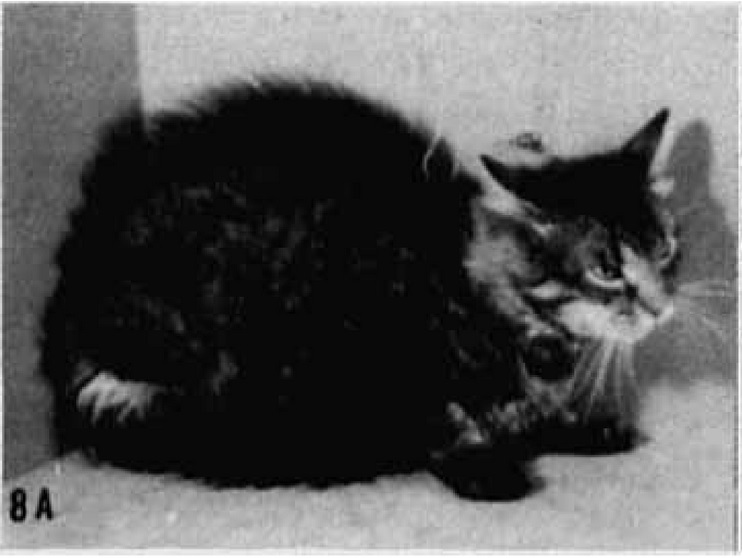
Piloerection
Piloerection (fur standing up) was seen in two cats on stimulation of points in the medial part of the basal nucleus of the amygdala.
Salivation
Salivation was obtained from the anteromedial part of the amygdala.
The stimulated cat secreted an abundance of thick and mucous saliva. The salivation was frequently associated with licking, swallowing, or chewing movements.
Other Brain Regions
Carl Sem-Jacobsen showed in human experiments on patients with schizophrenia or Parkinson's disease that various bodily functions occurred by electrical brain stimulation. (Sem-Jacobsen 1968)
The responses that occurred varied widely, including changes in breathing, taking a deep breath, coughing, sweating, nausea, vomiting, watery eyes, and the urge to urinate.
The majority of the responses came from the electrodes in the anterior part of the brain.
Changes in breathing were observed during stimulation, whereas taking a deep breath was observed most often at the end of the stimulation. (The nature of the response strongly indicates that apnea was actually induced.)
Patients who reported feeling nauseous sometimes vomited afterward, and the nausea often continued after the stimulation ended.
The urge to urinate was expressed as "Must go to the toilet." "Please, immediately."
But if the patient was then off the stimulation, he no longer felt the urge to urinate and volunteered remarks such as "No hurry." "You may finish, I'm in no hurry."
