Pleasure and Desire
Electrical stimulation of specific sites of the brain can cause pleasure and desire in humans and animals.
The sites that produce desire are along nerve fibers called the medial forebrain bundle and are widely distributed from the midbrain to the limbic system.
The sites that produce pleasure are fewer than those that produce desire and are scattered across limited brain regions known as the "pleasure center" or "hedonic hotspots."
If abused, this would make it possible to motivate individuals to take actions against their own wills.
In fact, there is a case where a man who had refused psychosurgery reversed his decision and agreed to undergo the procedure after receiving electrical stimulation to the lateral amygdala.
Table of ContentsAll_Pages
Circuitry
Pleasure
In the 1950s, it was discovered that rats with electrodes implanted in the septal area keep pressing a lever applying electrical stimulation there, compulsively repeating self-stimulation. (Olds and Milner 1954)
A man who repeated self-stimulation of the septal area behaved as if he was building up to an orgasm, stating that the feeling was “good.” (HEATH 1963)
A woman who had acetylcholine and norepinephrine injected in the septal area reached repetitive orgasms. (HEATH 1972)
This septal area became known as the "pleasure center."
Entering the 21st century, it was found that rats' hedonic facial expression to sweet tastes was amplified by injecting opioids into the nucleus accumbens, globus pallidus, insular cortex, and orbitofrontal cortex. (Morales and Berridge 2020)
These regions are known as "hedonic hotspots."
Of these, the nucleus accumbens is also an important region with respect to desire and is adjacent to the septal area.
The septal area and the nucleus accumbens are interconnected, and some researchers believe this contributes to the accumbal capacity to bring about hedonic impacts. (Zahm et al. 2012)
In addition, electrical stimulation of the amygdala, especially the lateral region, has been observed to produce pleasure responses in humans.
The ventral amygdalofugal pathway, one of the major output pathways of the amygdala, originates mainly from the central nucleus and basal (basolateral) nucleus in the lateral region of the amygdala, and connects the septal area and nucleus accumbens as well as the thalamus and hypothalamus. (Kamali et al. 2015)
This connection is likely what gives the amygdala its ability to produce pleasure.
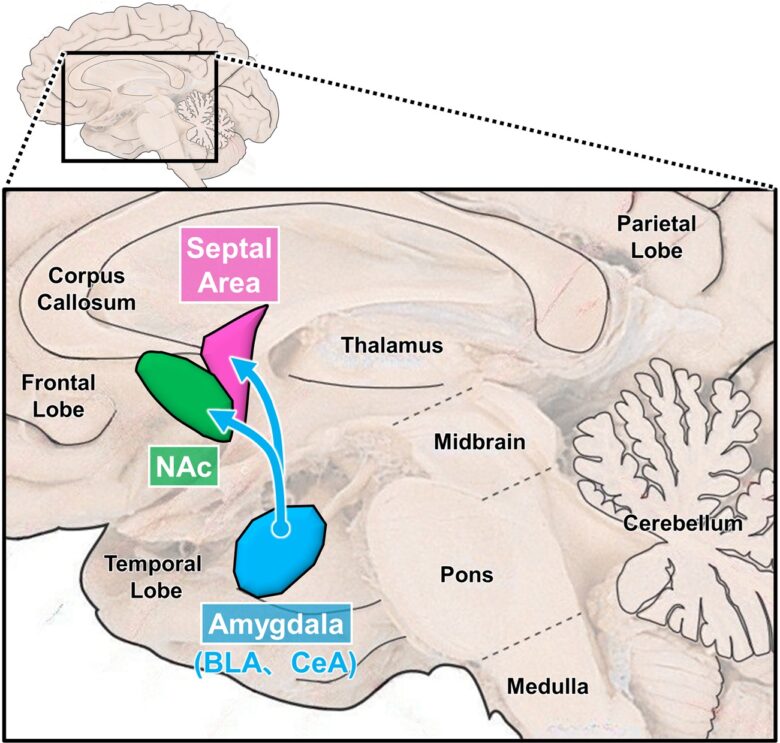
The septal area and the nucleus accumbens, two main pleasure-related brain sites, and the amygdala, their higher-level brain structure.
(the brain image from Vanderah 2018)

The septal area and the nucleus accumbens are adjacent to each other, with the septal area located on the midline and the nucleus accumbens on either side of it.
(the brain image from Vanderah 2018)
BLA = basal (basolateral) nucleus of the amygdala; CeA = central nucleus of the amygdala; NAc = nucleus accumbens.
Desire
Brain regions related to desire include the medial forebrain bundle (MFB), lateral hypothalamus, ventral tegmental area, nucleus accumbens, amygdala, and periaqueductal gray (PAG).
The MFB is a bundle of nerve fibers that runs through the lateral hypothalamus and is composed of no fewer than 50 descending and ascending fibers of varying lengths, origins, and terminations. (Nieuwenhuys et al. 1982)
Along these nerve fibers are located brain structures important for desire. (Wise 2005)
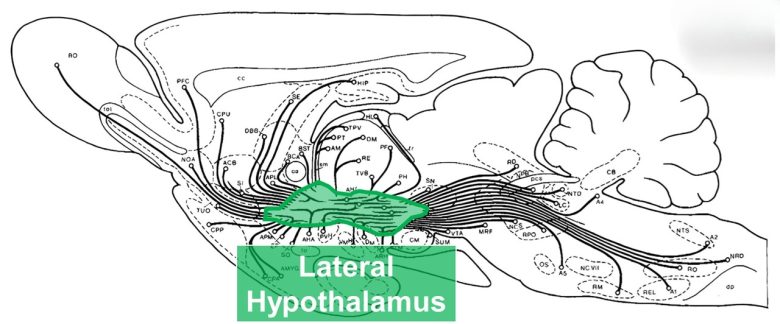
The MFB of the rat, consisting of numerous nerve fibers entering and exiting the lateral hypothalamus.
(Modified from Nieuwenhuys et al. 1982)
Electrical stimulation of the MFB in the lateral hypothalamus activates descending nerve fibers, which in turn activate the ventral tegmental area in the midbrain. (Sheehan et al. 2004)
Subsequently, dopamine neurons in the ventral tegmental area are activated, which conversely ascend and release dopamine into the nucleus accumbens, bringing about motivational effects.
It is widely believed that not only food, water, and sexual stimuli, but also drugs of abuse motivate humans and animals by stimulating the activity of these dopamine neurons.
The amygdala is a site at a higher level of the lateral hypothalamus, and the basal (basolateral) nucleus and central nucleus of the amygdala in particular have strong inputs to the lateral hypothalamus, controlling motivational behaviors such as feeding. (Reppucci and Petrovich 2015)
The ventral part of the PAG receives extensive input from the lateral hypothalamus, and activity in this region is necessary and sufficient for inducing exploratory, feeding, and predatory behaviors in animals. (Behbehani et al. 1988, Reis et al. 2024)
Electrical stimulation of the brain regions described above has been shown to increase motivation and appetite and to cause hypomania in humans, and to produce various motivational behaviors in animals, such as eating, drinking, preying, and mating.
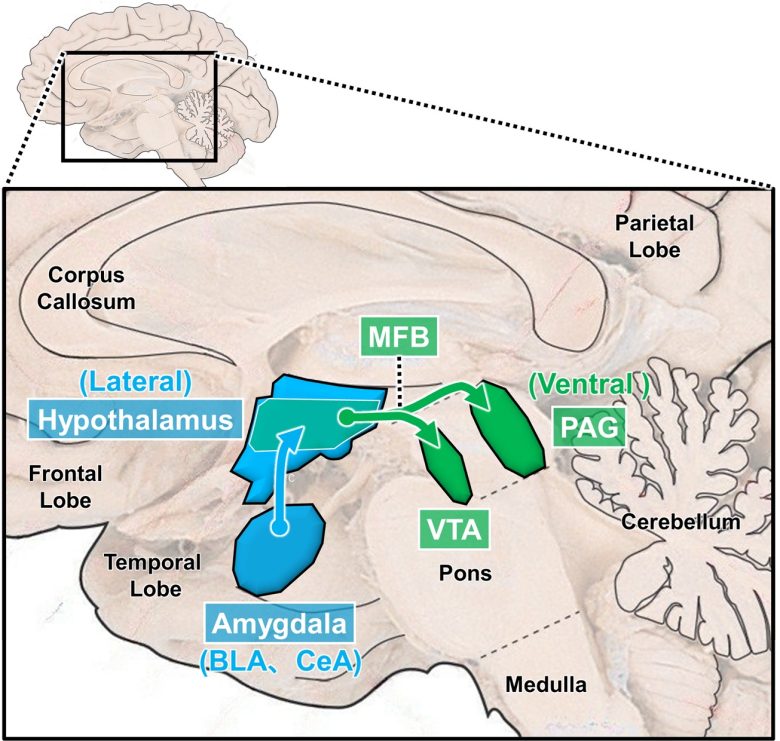
Descending desire circuit
(the brain image from Vanderah 2018)
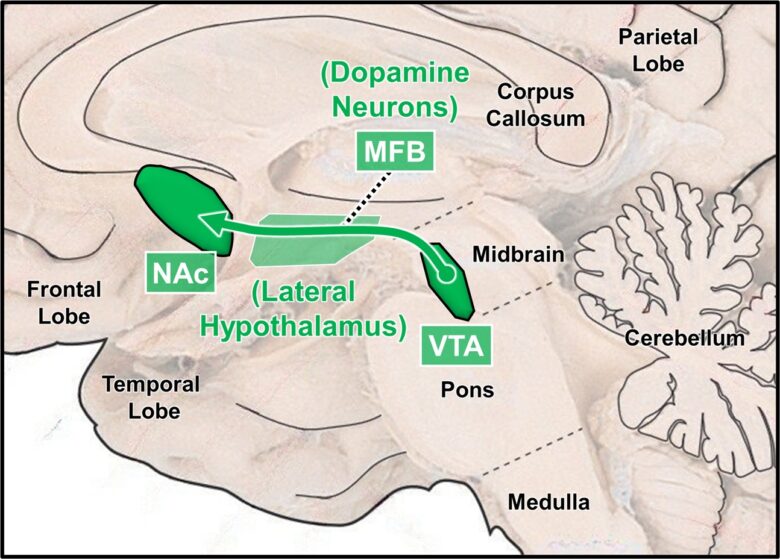
Ascending desire circuit (dopamine neurons)
(The brain image from Vanderah 2018)
BLA = basal (basolateral) nucleus of the amygdala; CeA = central nucleus of the amygdala; MFB = medial forebrain bundle; VTA = ventral tegmental area; PAG = periaqueductal gray; NAc = nucleus accumbens.
Desire Circuit vs. Rage/Fear Circuit
The following contrasts the desire circuit and rage/fear circuit I have explained so far.
This has turned out to be a generalization of the two types of attack circuits described on page 2: defensive rage and predatory attack.
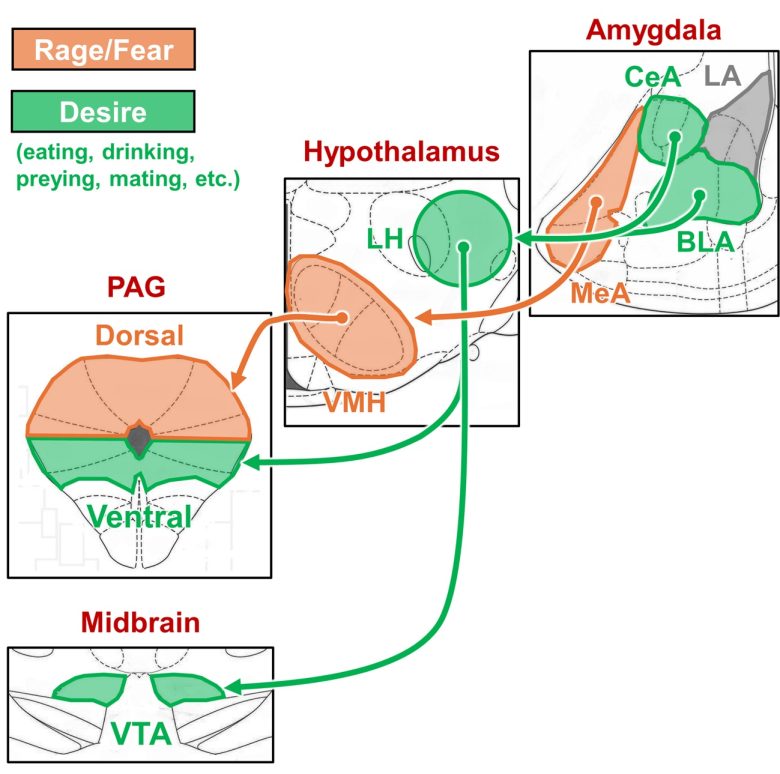
Desire circuit vs. rage/fear circuit
(The brain atlas from Paxinos and Watson 2009)
LA = lateral nucleus of the amygdala; CeA = central nucleus of the amygdala; BLA = basal (basolateral) nucleus of the amygdala; LH = lateral hypothalamus; VMH = ventromedial nucleus of the hypothalamus; PAG = periaqueductal gray; VTA = ventral tegmental area.
Two output pathways of the amygdala
The amygdala has two major output pathways called the stria terminalis (ST) and the ventral amygdalofugal pathway (VAP). (Isaacson 2012, Sun et al. 1991)
The ST mainly extends to the preoptic area, anterior hypothalamus, and ventromedial nucleus of the hypothalamus.
These nerve fibers arise primarily from the medial nucleus and cortical nucleus of the amygdala, with some contribution from the basal (basolateral) nucleus and central nucleus.
The VAP connects with the septal area, nucleus accumbens, thalamus, and lateral hypothalamus. (Kamali et al. 2015, Leonard and Scott 1971)
These nerve fibers arise primarily from the basal (basolateral) nucleus and central nucleus of the amygdala, with some contribution from the medial nucleus and cortical nucleus.
The ST outputs to the ventromedial nucleus of the hypothalamus, which is involved in fear and rage, mediating negative responses.
The VAP outputs to the lateral hypothalamus, which is involved in desire, and to the septal area and nucleus accumbens, which are involved in pleasure and desire, mediating positive responses.
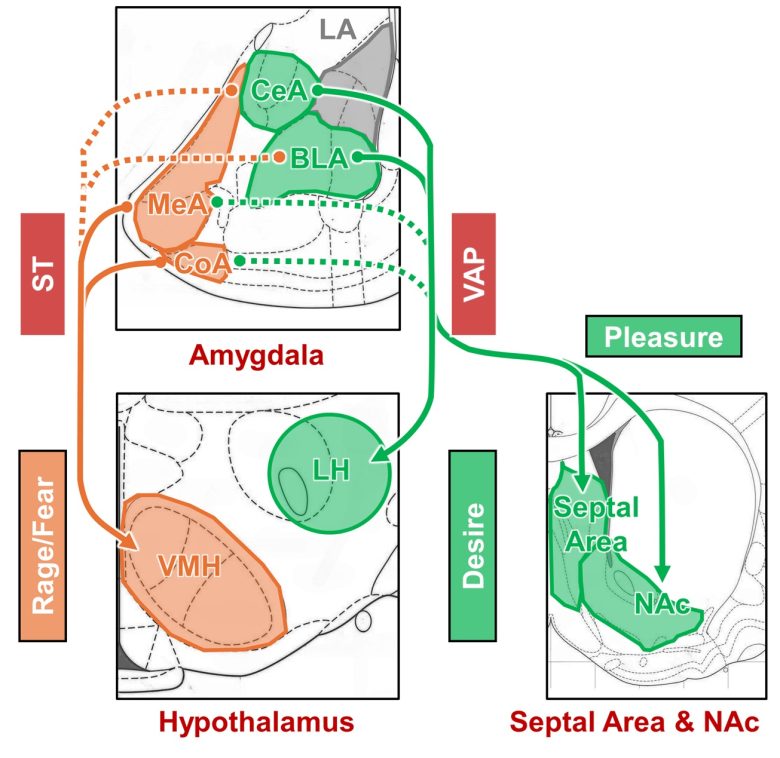
Outputs of the ST and the VAP to the hypothalamus
(the brain atlas from Paxinos and Watson 2009)
ST = stria terminalis; VAP = ventral amygdalofugal pathway; LA = lateral nucleus of the amygdala; CeA = central nucleus of the amygdala; BLA = basal (basolateral) nucleus of the amygdala; MeA = medial nucleus of the amygdala; CoA = cortical nucleus of the amygdala; LH = lateral hypothalamus; VMH = ventromedial nucleus of the hypothalamus; NAc = nucleus accumbens.
Upon electrical stimulation of the amygdala, it has been observed that positive responses such as happiness, euphoria, food intake, predation, and self-stimulation, as well as negative responses such as rage, fear, aggression, and flight, emerge from the same regions.
This may be explained in terms of which nerve fibers are activated: the ST or the VAP.
At least in humans, there is an actual case where electrical stimulation of the amygdala activated the VAP when pleasure was produced, and the ST when anger or anxiety was produced. (Avecillas-Chasin et al. 2020)
Humans
Electrical stimulation of the amygdala, septal area, and nucleus accumbens has been shown to produce pleasure.
Electrical stimulation of the medial forebrain bundle and nucleus accumbens has been shown to increase motivation and cause hypomania.
Electrical stimulation of the lateral hypothalamus has been shown to increase appetite.
Pleasure
Amygdala
Psychiatrist Robert Heath of Tulane University discovered, in human experiments with schizophrenic patients, that electrical stimulation of certain brain sites can produce pleasure. (Heath 1964)
Heath's favorite brain site for stimulation was the septal area, and his patients consistently reported sexual pleasure responses. (See page 5, "Sexual Behavior.")
Several patients also reported that electrical stimulation of the amygdala produced pleasure.
A 35-year-old male patient, "B-12," had numerous electrodes implanted in his brain, and it was arranged so that the patient could deliver electrical stimulation to his own brain by pressing a lever. (Bishop et al. 1963)
The patient was instructed to indicate whether electrical stimulation of each area brings about a pleasurable effect by the number of stimulation. (neither good nor bad: 3 times, bad: less than 3 times, good: as long as he wished)
The patient repeatedly self-stimulated the septal area, amygdala, head of the caudate nucleus, and hypothalamus, expressing that these brain sites were "good."
A 27-year-old female patient, "A-8," had electrodes implanted in the lateral amygdala. Low-intensity electrical stimulation of the lateral amygdala elicited pleasurable responses, and the patient often laughed. (HEATH et al. 1955, Heath 1996)
On the other hand, when the intensity of the electrical stimulation was increased so that the current spread to and activated the medial amygdala, it elicited rage responses that the patients could not resist.
Harvard psychiatrist Frank Ervin and neurosurgeon Vernon Mark observed a similar phenomenon. (Mark and Ervin 1970, Mark et al. 1972)
A 34-year-old male patient, Mr. Thomas R., suffered from episodes of violent aggressive behavior with feelings of intense hurt and depression.
When electrical stimulation was applied to the medial region of his amygdala, they observed a sign of the aggressive episode, so the stimulation was immediately stopped.
On the other hand, when electrical stimulation was applied to the lateral region of the amygdala, he obtained pleasurable sensations described as "tremendous feeling of relaxation relief."
These pleasurable sensations had a latency of 15-30 seconds and persisted for minutes to hours following cessation of the stimulation.
They were even able to obtain his consent for psychosurgery, which he had refused.
Other than that, a variety of transient mood or sensory changes occurred, most of which, in contrast to the said pleasure effects, were strictly limited to the duration of the electrical stimulation.
These changes were described as follows:
Left amygdala: "super-relaxed; pleasant dissociated; detached, tingling, confident."
Right amygdala: "pleasant, hopeful; relaxed; pleasure like demerol, confident; complete opposite of seizure; creative; elated; unreality; floating; decoupled; warm; peaceful; calm; natural, completion, satisfaction; deep thought; like going some new place with confidence for future."
There were no sexual or taste sensations, and no similar results were obtained in other brain sites.
Five separate electrical stimulations of this site by remote telemetry without warning induced typical relaxation and euphoria on a day when the patient was deeply depressed.
When the stimulation of this lateral region of the amygdala was discontinued for 10 days, the patient became irritable and severely depressed.
At a hospital in Marseille, France, an experiment was conducted to investigate the differences between the left and right amygdala in the emotional responses produced by electrical stimulation of the lateral region of the amygdala. (Lanteaume et al. 2006)
The subjects were eight epileptic patients, and had electrodes implanted primarily in the lateral nucleus and the basal nucleus of the amygdala.
Emotional responses induced by electrical stimulation of the left and right amygdala were quantified using a self-rated emotion scale.
They found that stimulation of the right amygdala increased only negative emotions. In contrast, stimulation of the left amygdala also induced positive emotions such as joy and happiness.
On the other hand, stimulation of the left amygdala also induced positive emotions such as joy and happiness.
Quantified Emotional Responses
UCLA researchers conducted an experiment to investigate the pathways activated, as well as the emotional responses produced, by electrical stimulation of the lateral amygdala. (Avecillas-Chasin et al. 2020)
The subjects were two PTSD patients, and had electrodes implanted primarily in the lateral nucleus and the basal nucleus of the amygdala.
Electrical stimulation of the basal nucleus of the amygdala induced positive responses such as happiness, euphoria, and relaxation.
This stimulation was confirmed to activate the ventral amygdalofugal pathway, which connects to the lateral hypothalamus, septal area, and nucleus accumbens.
In contrast, electrical stimulation of the lateral amygdala nucleus induced negative responses such as anger and anxiety.
This stimulation was confirmed to activate the stria terminalis, which connects to the medial hypothalamus.
Anger was also associated with relatively high stimulation intensity.
Nucleus Accumbens
Swiss psychiatrist Dr. Thomas Schlaepfer reported a case in which stimulation of the nucleus accumbens produced excessive euphoria. (Synofzik et al. 2012)
The patient was a 33-year-old German man diagnosed with combined generalized anxiety disorder and obsessive-compulsive disorder who had had the electrodes to the nucleus accumbens implanted at an outside medical center.
He visited the doctor's hospital for battery exchange of the stimulation device and adjustment of the stimulation parameters.
The parameter adjustment revealed two interesting phenomena.
First, a sensation of happiness and relaxation increased in proportion to the intensity of the stimulation.
With increasing intensity of stimulation, he sometimes likened the experience to the feeling of “getting high” or “being on drugs.”
The improvement in the patient's mood was also evident in the patient's hedonic behavior and a broad smile.
Second, at stronger stimulation, these sensations became excessive or “too much."
The patient began to feel “unrealistically good” and to be “overwhelmed” by the sensations of happiness and ease.
He became afraid that the positive effects will “tilt over” and that his “anxiety will come back.”
Based on these findings, an intermediate stimulation intensity was agreed upon, leaving the patient relatively happy and in a relaxed state, with neither anxiety nor an overwhelming feeling of happiness.
The next day, right before discharge, the patient asked to again increase the stimulation intensity, as he felt that he would like to feel “a bit happier” during the next weeks.
After considering risks such as addiction and ethical issues, the parameter settings were not changed.
Caudate Nucleus
Neurophysiologist Jose Delgado of Yale University reported a case in which euphoria was induced by electrical stimulation of the caudate nucleus. (Delgado et al. 1972)
The subject was a 35-year-old male patient with epilepsy who had a brain tumor in his temporal lobe.
Electrical stimulation of the head of the caudate nucleus significantly altered the patient's mood within 30 seconds.
Before the stimulation, he was reserved; his conversation was limited and he was concerned about his illness.
After the stimulation, the amount of his spontaneous speech increased more than twofold, with expressions of friendliness and euphoric behavior, which culminated in jokes and loud singing in a gay "cante jondo" style, accompanied by tapping with his right hand.
The euphoria continued for about 10 minutes and the patient then gradually reverted to his usual, more reserved attitude.
This was replicated in three different sessions.
Hypomania
Medial Forebrain Bundle (Near Subthalamic Nucleus)
Electrical stimulation of the subthalamic nucleus is a common treatment for Parkinson's disease, but it can cause hypomania/mania as a side effect.
Specific symptoms include abnormal mood elevation and increased activity, irritability, increased sexual desire, and delusional thoughts.
This side effect has been demonstrated to be caused by activation of the medial forebrain bundle extending from the subthalamic nucleus.
A 49-year-old male patient in Spain expressed euphoria two hours after starting electrical stimulation of the subthalamic nucleus. (Kulisevsky et al. 2002)
After 48 hours, the patient also showed pathological talkativeness, overactivity, and increased sexual drive.
He repeatedly asked why he had not been operated on earlier.
He demanded immediate discharge from the hospital because he “had never felt so good” and argued that he wanted to take a plane to complete a business deal.
The manic syndrome was persistent throughout the day. He also had grandiose delusions but no hallucinations.
After a few days, stimulation parameters were changed, overactivity and disturbances in mood partially improved, and the patient was discharged 10 days after surgery without mood stabilizers.
After discharged from the hospital, the hypomania resolved within a few days.
A 65-year-old female patient in Germany showed a remarkable mood change after starting electrical stimulation of the subthalamic nucleus. (Herzog et al. 2003)
In parallel with motor improvement, her mood was elevated to a degree that was abnormal for the patient.
She was excessively talkative and it was not possible to interrupt her while she was speaking. Gradually, in the course of the next 3 weeks, hypomania turned into a manic episode with psychotic symptoms.
The patient’s mood was euphoric, her speech was more rapid, her thought was easily distracted, flights of ideas appeared, associations loosened, and her ability to concentrate faded.
She lost normal social inhibitions, was in love with two neurologists, and tried to embrace and kiss people.
She was hyperactive and restless, she left the clinic several times without permission and engaged in unrestrained buying of clothing. Because of her disorganized behavior she messed up her room, and she occupied her neighbor’s bed.
The family wanted to remove her credit card to protect her from financial ruin. Her judgment was impaired, but she had little insight into her disorder and had low frustration tolerance.
She was suspicious, tense, and hostile, and had the delusion that her sons were conspiring against her and said that they tried to get her money by threat of force.
Combined with medication, the affective disorder eventually resolved.
A 59-year-old male patient in Sweden was pre-operatively a very quiet and taciturn person without any history of psychiatric disorders. (Kim et al. 2012)
After starting electrical stimulation of the subthalamic nucleus, his motor disability improved rapidly and markedly.
However, a remarkable mood change took place 2 days after.
His mood was elevated, and he became talkative and excessively hopeful.
He danced up and down and sang with other patients in the neurosurgical ward, and furthermore, had sexual interest increased.
A research group at the University of British Columbia reported three cases in which electrical stimulation of the subthalamic nucleus produced mania. (Mandat et al. 2006, Coenen et al. 2009)
The first patient was a 72-year-old man, and electrical stimulation improved his motor function significantly.
He reported no adverse effects and mentioned an increased sense of well-being which he attributed to his new mobility.
However, he purchased a new car, even though he couldn't drive due to his deformed feet.
He also arranged for a prostitute to visit his nursing home. This behavior was not at all in keeping with his personality nor the rules at his facility.
After stimulation parameters were changed, hypomanic behavior disappeared and the motor improvements remained.
With changed stimulation parameters, they were able to resolve hypomania while maintaining improved motor function.
The second patient was a 45-year-old man, and again electrical stimulation improved motor function significantly.
He reported no adverse effects and mentioned he felt more awake and alert.
However, the patient broke into a parked car in the middle of a crowded street. He was unable to explain why he did this.
He remembered that during the incident he felt "over the top and invincible." He did not feel anger while breaking into the car.
He did not feel any stress about the resultant upcoming court.
With changed stimulation parameters, the feeling of invincibility faded away in 3–4 days and he became appropriately anxious about his court proceedings.
After that, the hypomanic behavior was no longer observed.
The third patient was a 66-year-old man, and once electrical stimulation of the subthalamic nucleus was initiated, the patient showed an increased ability to walk around.
However, after 10 days, the patient began to exhibit extraordinary behavior.
The patient had started to wear his wife’s clothes, demanded sexual intercourse daily, and showed increased risk-taking behavior, particularly reckless driving.
The patient was admitted to the hospital. He appeared agitated and understood that his behavior was wrong, but he explained that “something was driving” him.
Stimulation was shut off and his urges stopped almost instantaneously.
A year previously, while taking the dopaminergic medication called pramipexole, a similar but less-pronounced sexual fetish behavior had transiently occurred as well.
With the use of a more superficial electrode contact, his motor symptoms improved and the hypomanic behavior disappeared.
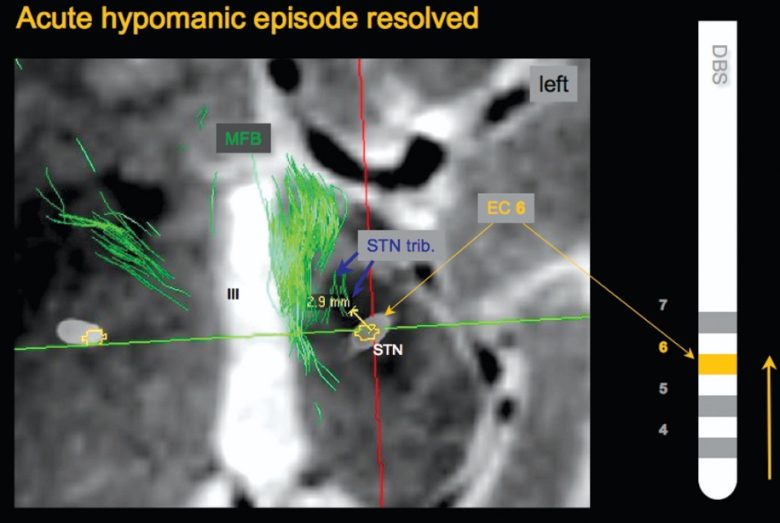
The research group investigated why electrical stimulation of the subthalamic nucleus sometimes causes patients to develop hypomania. (Coenen et al. 2009)
When they visualized the nerve fibers in the patients' brains, they found that in a patient who developed hypomania, the electrode contact was in direct contact with the medial forebrain bundle.
On the other hand, patients who did not develop hypomania had electrode contacts located further away from the medial forebrain bundle.
The medial forebrain bundle that the electrode was in contact with was not the mainstream originating from the well-known ventral tegmental area, but rather tributaries originating from the subthalamic nucleus.
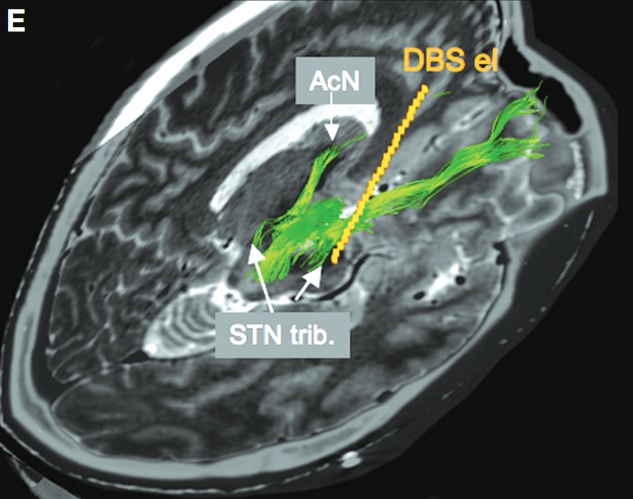
When the electrode contact of the patient who had developed hypomania was changed one step up so that it did not contact the medial forebrain bundle, the hypomania resolved and the motor symptoms further improved.

Nucleus Accumbens
Electrical stimulation of the nucleus accumbens has been experimentally used to treat obsessive-compulsive disorder, but has been observed to cause hypomania in patients as a side effect.
In a collaborative study by Brown University School of Medicine and the Cleveland Clinic, electrical stimulation of the internal capsule just above the nucleus accumbens produced hypomania in 5 out of 10 subjects. (Greenberg et al. 2006)
Within seconds of stimulation, there appeared in the patients a transient elevated mood associated with noticeably increased energy, speech production, and spontaneity of social interactions.
In a study at the University of Cologne in Germany, electrical stimulation of the nucleus accumbens produced hypomania in 2 out of 10 participants. (Huff et al. 2010)
One patient reported concentration difficulties and failing memory.
In a study at the University of Amsterdam, electrical stimulation of the nucleus accumbens produced hypomania in 8 out of 16 participants. (Denys et al. 2010)
Increased libido was reported by 7 patients, but this was not experienced as uncomfortable.
Increased Motivation
Nucleus Accumbens
One of the symptoms of depression is a decrease in motivation, but electrical stimulation of the nucleus accumbens has been shown to increase motivation in patients.
Swiss psychiatrist Dr. Thomas Schlaepfer administered electrical stimulation to the nucleus accumbens to three patients as an experimental treatment for depression. (Schlaepfer et al. 2007)
Two patients experienced an immediate and rapid increase in motivation.
The first patient spontaneously reported immediately after stimulation that he had never visited the famous Cologne Cathedral and planned on doing this in the immediate future.
The second patient spontaneously mentioned immediately after stimulation that she wished to take up bowling again, which was a favorite pastime of hers 12 years ago, before the onset of her depression.
She noted, "This would be quite pleasurable."
This is especially noteworthy given these patients' severe lack of motivation during their long depressive episode.
Depression symptoms improved in all three patients with electrical stimulation.
Next, the doctor examined the long-term therapeutic effects of electrical stimulation of the nucleus accumbens. (Bewernick et al. 2012)
In 5 of the 11 patients, there appeared improvement in their symptoms, and they constantly demonstrated increased motivation.
The patients started to work part-time, took care of their personal matters, resumed new hobbies, one patient developed the desire to have a child, and so on.
Two patients presented with transient hypomanic symptoms of elevated mood and fewer hours of sleep.
Medial Forebrain Bundle
Dr. Schlaepfer also administered electrical stimulation of the medial forebrain bundle to seven patients as an experimental treatment for depression. (Schlaepfer et al. 2013)
During intra-operative test stimulation, all patients consistently showed increased motivation and mood improvement.
An increase in motivation was indicated by orientation reaction, initialization of eye contact, engaging in conversation with the psychologist, etc.
Shortly after starting continuous electrical stimulation, six patients reported acute changes in mood, anxiety, and drive, and after a few months, four people were diagnosed with remission from depression.
Increased Appetite
Hypothalamus
At a hospital in Copenhagen, Denmark, electrical coagulation of the lateral hypothalamus was applied as an experimental treatment for obesity. (Quaade et al. 1974)
The subjects were four women and one man, and all were hyperphagia and had a pronounced obesity, weighing between 260 and 397 lb (118 and 180 kg).
Prior to the coagulation, an experiment was conducted on electrical stimulation of the lateral hypothalamus.
They found that 3 out of 5 people showed convincing hunger responses.
For example, the following statements were seen:
"I am so hungry that I could eat a whole fried chicken with chips."
"I am so hungry that my entire belly feels as a vacuum."
Other than that, fear was obtained from the lower part of the electrode track, whereas intense pleasure, bordering on euphoria, was obtained from the upper part.
Coagulation was then conducted at the site where the hunger responses were induced.
One week after surgery, the patients stated that for the first time they were not very hungry and, especially, felt satiated already during the beginning of their meals.
Patients' body weight was slightly and temporarily decreasing, but not significantly affected.
One patient, after her second coagulation, showed a slight reduction of spontaneity and initiative. This lasted for 2-3 months, after which she went back to her normal mental state.
Animals
Animals compulsively self-stimulate brain regions along the medial forebrain bundle, including the septal area, amygdala, nucleus accumbens, lateral hypothalamus, ventral tegmental area, and periaqueductal gray.
Animals repeatedly engaging in such self-stimulation have also been observed to exhibit symptoms similar to those of drug addiction.
In addition, electrical stimulation of these brain regions can cause animals to take on various motivated behaviors, such as food intake, water intake, and predation.
These effects can, for example, have well-fed animals eat forcibly, causing them to become obese.
Self-Stimulation
Septal Area
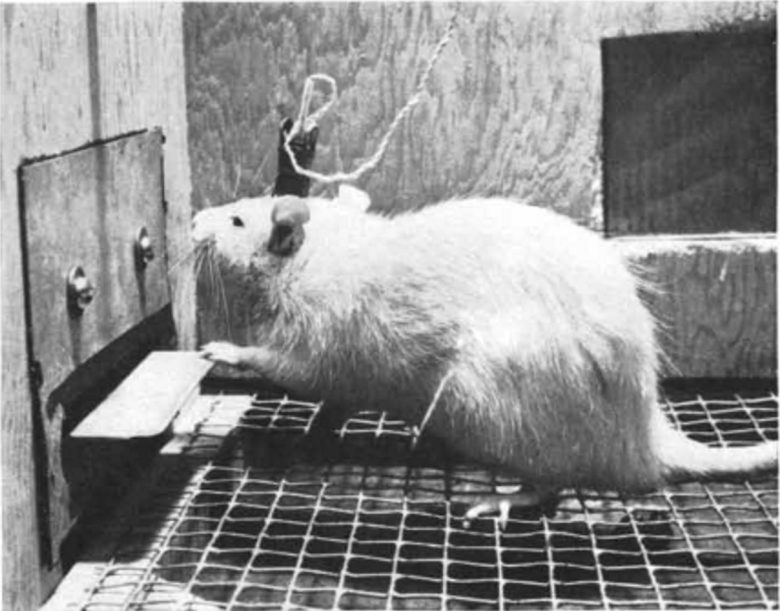
In 1954, American psychologist Dr. James Olds discovered that a rat with an electrode implanted in the septal area would compulsively self-stimulate by repeatedly pressing a lever that delivered electrical stimulation there. (Olds and Milner 1954)
Animals who were self-stimulating the septal area appeared outwardly to be relishing a highly satisfying pleasure. They periodically shivered, as if their experience was remarkably intense. (Panksepp 1998)
This effect could also be used to guide rats to specific locations. (Olds 1956)
For example, by applying electrical stimulation to the septal area every time the rat went to a certain corner of the cage, the doctor was able to keep it in that corner.
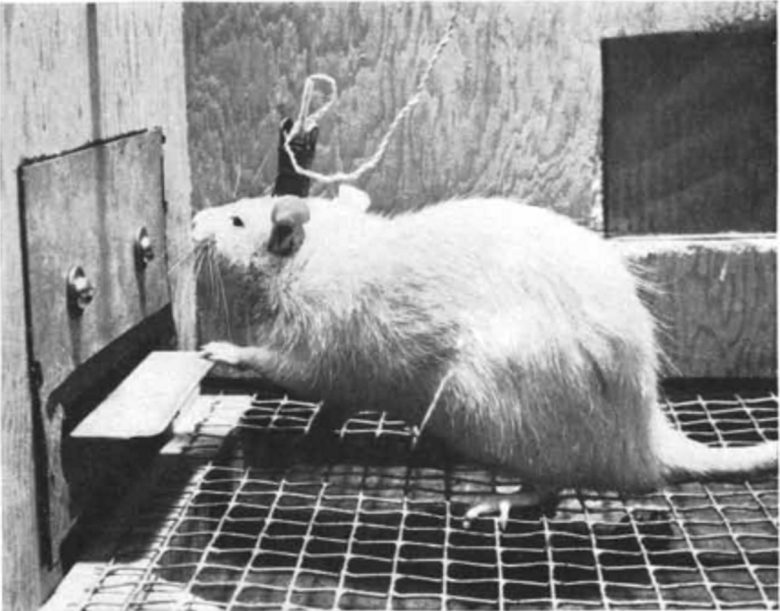
The septal area came to be called the "pleasure center."
This finding has been replicated many times in rats, and other species have been shown to self-stimulate their brains as well, including goldfish, guinea pigs, bottlenose dolphins, cats, dogs, goats, and monkeys. (Bishop et al. 1963)

A monkey continues to stimulate its brain by pressing the lever with its left hand while restrained in the chair. (Brady 1961)
Surprisingly, brain self-stimulation experiments were also carried out on humans.
Subjects who self-stimulated the septal area consistently reported experiencing sexual pleasure or sexual desire. They even went into a convulsion from the strong pleasure at times. (See page 5, "Sexual Behavior.")
Medial Forebrain Bundle
Dr. Olds wondered if there were other brain regions that rats would self-stimulate besides the septal area and searched for them. (Olds 1956, Olds and Olds 1963)
They were found in a wide range of regions, including the hypothalamus, amygdala, nucleus accumbens, anterior cingulate cortex, hippocampus, and ventral tegmental area.
Among these, what rats self-stimulated most intensely were the regions of the diencephalon and midbrain along the medial forebrain bundle, extending from the lateral hypothalamus to the ventral tegmental area.
Electric stimulation of these regions appeared to be far more rewarding to the animals than an ordinary satisfier such as food. (Olds 1956)
For example, hungry rats ran faster to reach an electrical stimulator than they did to reach food. Indeed, a hungry rat often ignored available food in favor of the pleasure of stimulating itself electrically.
Some rats stimulated their brains more than 2,000 times per hour for 24 consecutive hours.
Well-fed rats running for a brain-stimulation reward endured electric foot shock on the way to the reward far more than did 24-hour-hungry rats running for food reward. (Olds 1958)
Lateral Hypothalamus
Researchers at Cornell University compared the natures of self-stimulation in rats obtained from the septal area and lateral hypothalamus (LH). (Spies 1965)
In the experiment, electrodes were placed in either the septal area or LH in rats to investigate whether they preferred self-stimulation or food.
Two levers were placed inside the cage: one for brain self-stimulation and the other for dispensing food.
The rats were allowed to both self-stimulate and eat during a 1-hour session.
They found that the LH rats continued to choose self-stimulation despite being hungry, ending up starving themselves.
On the other hand, the septal rats continued to self-stimulate while also eating food and maintaining their weight.
Furthermore, among the LH rats, those whose electrode contacts weren't in contact with the medial forebrain bundle (MFB) exhibited the same behavioral pattern as the septal rats and did not end up starving themselves.
Body Weight
The LH + MFB rats continued to choose self-stimulation despite being hungry, ending up starving themselves. On the other hand, the septal rats continued to self-stimulate while also eating food and maintaining their weight.
Self-Stimulation and Food Intake
The LH + MFB rats self-stimulated intensely despite being hungry, whereas the septal rats self-stimulated while also eating food.
Ventral Tegmental Area
Researchers at the University of Bordeaux in France compared the natures of self-stimulation in rats obtained from the ventral tegmental area (VTA) and the lateral hypothalamus (LH). (Miliaressis and Cardo 1973)
A self-stimulation lever and a food pellet lever were each placed in the cage. The rats were allowed to both self-stimulate and eat during a 30-minute session.
The experiment was conducted with varying degrees of food deprivation, from the rats being satiated to deprived of food for 24, 48, and 72 hours.
The rats with electrodes placed in either the VTA or the LH both self-stimulated intensely despite being hungry.
However, as the deprivation period became longer, the amount of food intake increased.
There was a general tendency that the VTA rats self-stimulated slightly more and the LH rats ate food slightly more.
Self-Stimulation and Food Intake
by Degrees of Deprivation
A characteristic tendency observed during food deprivation was that the VTA rats progressively abandoned food intake and shifted to self-stimulation.
On the other hand, LH rats showed the opposite tendency, progressively abandoning self-stimulation and shifting to food intake.
Self-Stimulation and Food Intake
During Food Deprivation
PAG
UCLA researchers discovered that consistent and vigorous self-stimulation can be obtained from the ventral part of the periaqueductal gray (PAG). (Liebman et al. 1973)
The rate of self-stimulation of rats is extremely high, at over 6,000 times per hour, and vigorous sniffing often accompanied self-stimulation.
On the other hand, the rats did not self-stimulate the dorsal part of the PAG at all, and it was obvious that the stimulation was aversive judging from behavioral signs.
As a matter of fact, the dorsal part of the PAG is known to be the region that induces rage and fear responses. (See page 2, "Rage," and page 3, "Fear.")
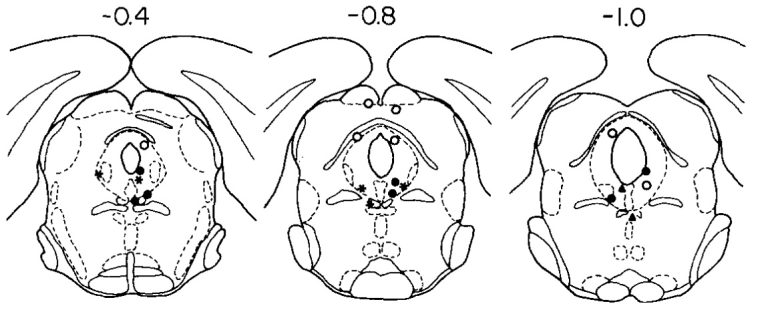
Black circle = high rate; black star = moderate rate; white circle = none/low rate.
Nucleus Accumbens
Researchers from the University of Sydney compared the natures of self-stimulation in rats obtained from the nucleus accumbens and lateral hypothalamus (LH). (Jenkins et al. 1983)
The acquisition of vigorous self-stimulation was very rapid in the LH rats, taking only 3 days.
On the other hand, it took more than 20 days for the accumbal rats to acquire comparable vigorous self-stimulation.
Afterwards, however, the accumbal rats show more compulsive behavior, and the absence of stimulation-bound sniffing, rearing and exploration was quite marked.
In addition, after one day of deprivation of self-stimulation, the accumbal rats rushed to the stimulation device and the time it took them to start stimulation was considerably reduced.
Furthermore, the rats gradually developed seizures over the three weeks of the study, and subsequently, the seizures became more frequent and severe.
Wet-dog shakes, a characteristic of morphine withdrawal syndrome, were also observed.
These phenomena were thought to be due to changes in the opioid system in the nucleus accumbens.
That is, the rats that repeatedly self-stimulated the nucleus accumbens exhibited symptoms similar to those of drug addiction.
Dopamine Neurons
A study at the University of Geneva in Switzerland discovered that continuous activation of dopamine neurons produces behavioral and cellular abnormalities in the rats that are similar to those seen in drug addiction. (Pascoli et al. 2015)
First, they placed a lever in the cage that directly activated dopamine neurons in the ventral tegmental area in the rats with laser light.
The rats were then placed in the cage, and they quickly learned to press the lever and self-stimulate their own dopamine neurons.
Even when the rats received an electric foot shock after each stimulation, they did not stop self-stimulating.
The electric foot shock was strong enough to completely suppress lever pressing for a sweet water reward.
The rats were allowed to use the self-stimulation device for 12 consecutive days, and then after 30 days of "abstinence," the rats were exposed to the same device again.
They found that the rats then sought self-stimulation and kept pressing the lever of the device that had been turned off, exhibiting behavior similar to withdrawal symptoms.
Neuronal changes in the nucleus accumbens of the withdrawal rats were indistinguishable from those observed after withdrawal from cocaine self-administration.
Amygdala
Dr. Olds was surprised to find that animals did self-stimulate the amygdala, which was well known at the time for its involvement in negative emotions such as rage and fear, so he investigated this further. (Wurtz and Olds 1963)
Electrodes were implanted in a wide region of the amygdala in rats to examine whether they produced either self-stimulation or escape responses.
The doctor found that self-stimulation occurred mostly from the central nucleus and medial nucleus, while escape occurred mostly from the lateral nucleus and basal nucleus.
However, the responses were not strictly localized, and the distribution of self-stimulation and escape overlapped, with escape sometimes occurring from the medial nucleus and self-stimulation sometimes occurring from the basal nucleus.
Recent studies using precise laser stimulation have shown that further subdivision of the regions allows opposing behaviors obtained from the amygdala to be mapped to topographically distinct regions. (Kim et al. 2016, Kim et al. 2017)
In the experiments, they further subdivided the basal nucleus and central nucleus (Ce) of the amygdala and showed that self-stimulation and freezing of rats can be induced separately from these subregions.
Freezing was obtained from the magnocellular neurons in the anterior basal nucleus, and self-stimulation was obtained from the parvocellular neurons in the posterior basal nucleus.
Of the central nucleus, freezing was obtained from the capsular nucleus (CeC), and self-stimulation was obtained from the medial nucleus (CeM) and lateral nucleus (CeL).
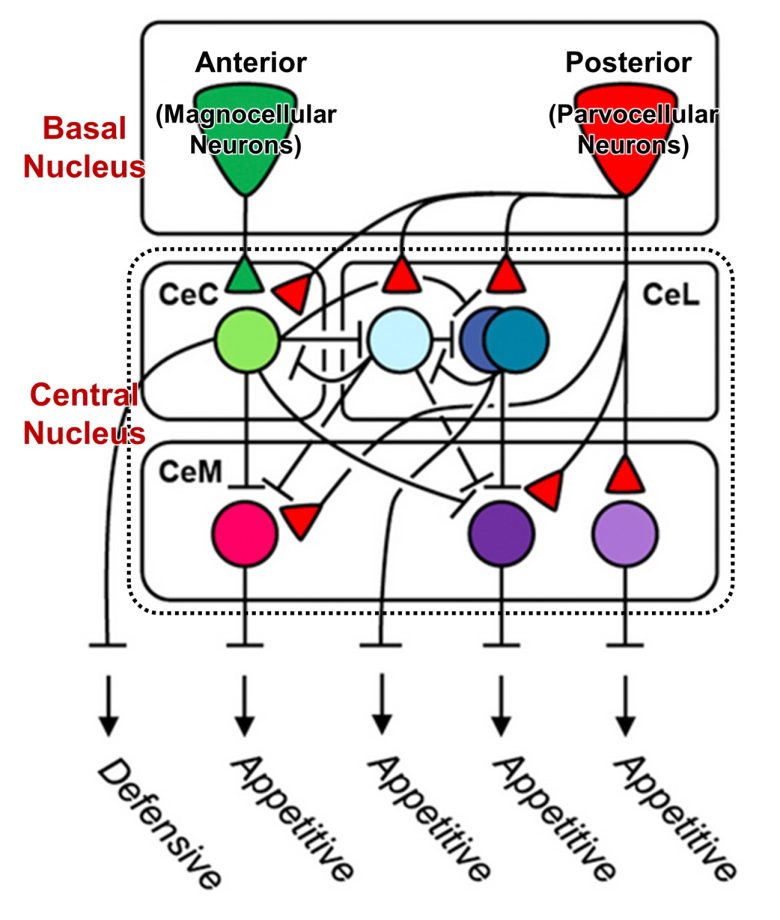
Food Intake
The induction of feeding behavior is mainly confirmed in the lateral hypothalamus, sometimes called the “hunger center.”
It has also been observed in the amygdala, medial forebrain bundle, septal area, periaqueductal gray, nucleus accumbens, etc., which overlap with brain regions that animals self-stimulate.
Lateral Hypothalamus
Dr. Walter Hess of the University of Zurich in Switzerland discovered that electrical stimulation of the lateral hypothalamus in cats produces binge eating. (Hess 1958)
The cat had previously taken neither milk nor meat, but then devoured or drank greedily upon the stimulation.
As a matter of fact, the animal might even take into its mouth or gnaw on objects that are unsuitable as food, such as forceps, keys, or sticks.
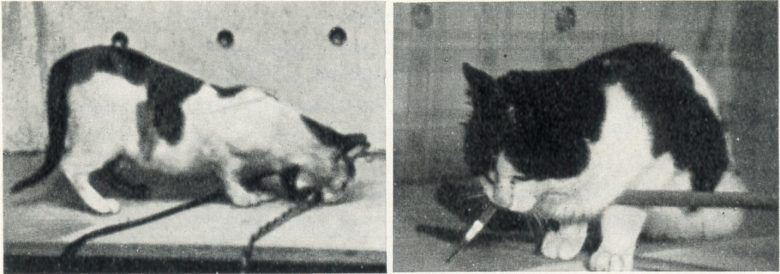
Japanese researchers also showed that electrical stimulation of the lateral hypothalamus in cats produces binge eating. (UMEMOTO and KIDO 1967)
The cat could take food (boiled fish and rice) in a small vessel set in the cage at any time and was well-fed.
Upon electrical stimulation of the lateral hypothalamus, the cat approached the food vessel with sniffing and ate fish energetically.
These responses ceased immediately when the stimulation turned off. At this time, for several seconds, the cat showed the freezing posture.
On increasing the stimulation intensity, the responses became more violent, and the cat could not discriminate between edible and inedible objects, and bit the food vessel.
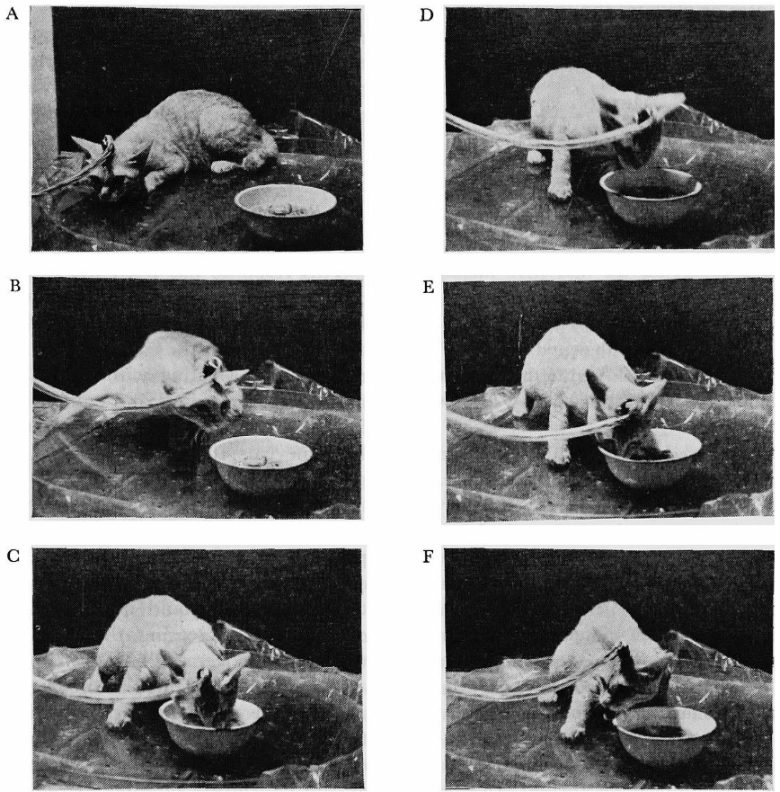
A: Before stimulation; B-E: During stimulation; F: Immediately after stimulation ended.
Dr. Neal Miller of Yale University furthered binge eating and succeeded in making rats obese through long-term electrical stimulation of the lateral hypothalamus. (Steinbaum and Miller 1965)
In the experiment, electrical stimulation of the lateral hypothalamus was continuously delivered to the rats for two hours per day for 31 days.
The doctor found that from the eighth day, the rats began to overeat during the stimulation period and eventually became obese.
At the end of the experiment, the stimulated rats were consuming about 2.5 times the amount of food per day that the normal control rats ate.
Food Intake and Body Weight
Rats were observed to be bloated with food and completely inactive in between the stimulations, and spent most of their time on their backs with limbs in the air.
The stomachs of these rats were found to be unusually distended, and there was hemorrhage in some rats.
When the stimulation stopped, the rat's food intake significantly decreased to 10% of normal levels in an attempt to compensate for the overeating.
However, the rat was subsequently forced to overeat by brain stimulation to an extent that it could not compensate, so its free-will feeding behavior was completely stopped, causing the rat to become obese.
In opossums as well, electrical stimulation of the lateral hypothalamus has been shown to produce food intake. (Roberts et al. 1967)
The eating behavior was similar to that seen in normal eating.
The food was seized by the incisors and canines, shifted to the back of the mouth, masticated, and usually swallowed.
The forepaws were usually employed to push food back into the mouth when it began to fall out, but in a few cases a high percentage of the food was allowed to fall out of the mouth during the chewing.
Amygdala
Stimulation of the amygdala has been shown not only to induce food intake but also to facilitate it. (Montgomery and Singer 1975)
In this experiment, instead of electrical stimulation, chemical stimulation was performed by injecting norepinephrine, a neurotransmitter, into the medial nucleus of the amygdala and lateral hypothalamus (LH).
First, they confirmed that stimulation of the LH increased food intake in satiated rats.
They then found that additional stimulation of the cortical nucleus of the amygdala augmented this increase in food intake.
Amygdala stimulation alone increased food intake, but to a lesser extent than LH stimulation.
Amount of Food Intake
Amygdala (AMY) stimulation facilitated food intake induced by LH stimulation.
Ventral Tegmental Area
Electrical stimulation of the ventral tegmental area has also been shown to induce food intake in rats. (Waldbillig 1975)
The responses were similar to those induced from the hypothalamus.
The feeding behavior consisted of the sequence of seizing food with the incisors, transferring it to the paws, biting off a small morsel, masticating, and swallowing.
The responses were never directed to inappropriate objects.
Septal Area
Electrical stimulation of the septal area has also been shown to induce food intake in rats. (Altman and Wishart 1971)
The stimulated rat performed a behavioral sequence in which shaking occurs first, followed by rather extensive grooming, and culminating in the ingestion of food if available.
It appeared that the feeding behavior by stimulation of the septal area was not as intense as that by stimulation of the lateral hypothalamus or as that by natural hunger.
Nucleus Accumbens
Electrical stimulation of the nucleus accumbens has also been shown to induce food intake in rats. (van der Plasse et al. 2012)
The nucleus accumbens is divided into two structures, the shell and the core, but food intake was induced only by electrical stimulation of the medial part of the accumbal shell.
In addition, as the intensity of the electrical stimulation increased, the amount of food intake in rats increased.
Amount of Food Intake
Medial Forebrain Bundle
The Yerkes National Primate Research Center conducted an experiment to investigate the regions that induce food intake by implanting numerous electrodes into the brains of rhesus monkeys. (Robinson and Mishkin 1968)
Stimulation that induced responses with the lowest intensity was the stimulation of the medial forebrain bundle, centered on the lateral hypothalamus and extending from the medial preoptic area to the ventral tegmental area.
In typical responses, the monkeys took food held close to their mouths 2–5 seconds after the onset of electrical stimulation.
In intense responses, the food was rapidly delivered to a cheek pouch and then more food was accepted until both pouches and the mouth were full and it became physically impossible for the monkey to receive more.
In less intense responses, the food was kept only in the mouth.
Regardless of the intensities of the responses, the received food was rarely chewed and swallowed during stimulation but was regularly ejected from the pouches and mouth within 3-15 seconds following cessation of the stimulation.
These behaviors were reproduced with each repeated stimulation.
Intake of non-food objects also occurred, and the behavior closely resembled intake of food.
In intense responses, the mouth and pouches could be filled to capacity with corks and miscellaneous objects, and none of these objects was ever swallowed.
Predation
Here I will present examples in which feeding and preying result from electrical stimulation of the same brain region.
For other predatory attack experiments, see page 2, "Rage."
Lateral Hypothalamus
An experiment at an Illinois psychiatric hospital showed that electrical stimulation of the lateral hypothalamus in cats can produce not only food intake but also predatory attack toward rats. (Hutchinson and Renfrew 1966)
First, one hour before the experiment began, the cats were fed their entire daily food ration.
The cats were then placed in the cages with rats, and the cats did not voluntarily attack the rats.
Here, electrical stimulation of the lateral hypothalamus in the cats caused them to launch predatory attack on the rats.
The cat crouched close to the cage floor, crept up to the rat, and grasped the head or neck in its jaws while pinning the rat to the floor with one paw.
The attack was silent, with the pupil dilated, fur standing up restricted to the tail, and increased respiration.
Next, a bowl of horse meat was placed in the cage instead of the rat.
Here, electrical stimulation of the lateral hypothalamus in the cats caused them to begin eating the meat.
The cat approached the dish 15-20 seconds after the stimulation, and ate for the duration of the stimulation.
When stimulated in the presence of both the rat and the horse meat, both predatory attack and food intake were induced in the cats.
Whether to prey on a rat or feed on horse meat depended on which target was contacted first.
If the rat was placed closer to the cat, the rat was attacked by the cat.
On the contrary, if the bowl was placed closer, the horse meat on the bowl was eaten.
PAG
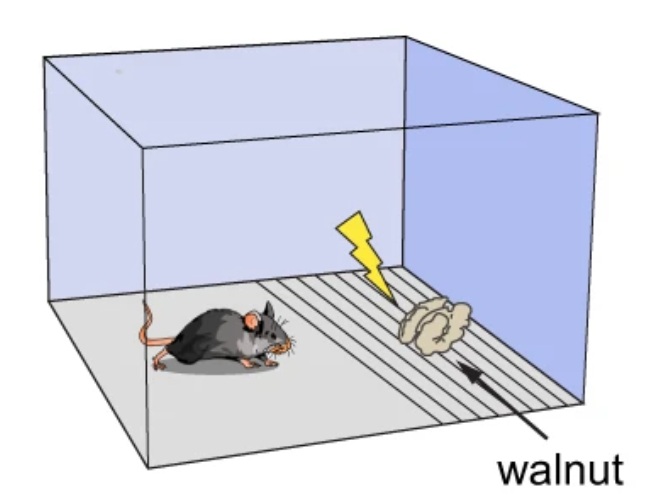
UCLA researchers showed that stimulation of the ventral part of the periaqueductal gray (PAG) in mice results in exploration, food intake, and predation. (Reis et al. 2024)
The presence of nothing in the cage induced exploration, the presence of walnuts induced food intake, and the presence of crickets induced predation.
Placing an electric shock grill in the path to access walnuts reduced walnut intake.
However, the mice that received stimulation of the ventral part of the PAG showed walnut intake comparable to that in the absence of the shock grill.
When they attached a ball to a long stick and moved the ball in a trajectory that spelled the letters “BG,” the mice that received stimulation of the ventral part of the PAG relentlessly followed the ball.
The mice that did not receive stimulation did not exhibit this behavior.
This pursuit of the ball was very similar to the relentless pursuit of crickets induced by laser stimulation of the ventral part of the PAG.
Water Intake
Lateral Hypothalamus
The National Cancer Institute found that electrical stimulation of the lateral hypothalamus in rats can produce water intake. (Greer 1955)
It was immediately apparent that the stimulated rat was under great compulsion to perform violent “licking” activity.
The rat stood on its hind legs and ran vigorously around the glass-enclosed circular cage, licking wildly at the glass wall.

This behavior ceased immediately upon shutting off the stimulation. As the stimulation intensity was gradually increased, the licking behavior became progressively more intense.
When the water bottle was present, licking behavior changed to drinking behavior. As soon as the switch was turned on, the rat jumped for the water bottle and continued to drink avidly until the switch was turned off.
When the tap water in the drinking bottle was replaced with saline, the rat began by drinking as vigorously as before, but it was obvious that the saline was quite distasteful.
The rat pushed at the water spout with its forepaws and finally was able to force itself away from the bottle for a few seconds, then the compulsion to drink again became too great and it would be forced back to lapping at the saline.
When it was being continuously stimulated five minutes every half hour, the rat drank in excess of 0.7 pt (0.4 L) of tap water daily.
Dr. Neal Miller of Yale University showed that electrical stimulation of the lateral hypothalamus can force rats to drink milk laced with a bittering agent. (Tenen and Miller 1964)
First, the rats were given food, water, and milk until they were satisfied, and then the doctor confirmed that electrical stimulation of the lateral hypothalamus caused the rats to begin drinking milk again.
In general, all the rats drank the milk for the full 15 seconds of stimulation and stopped drinking at the termination of the stimulation.
Next, the milk was laced with the bittering agent quinine.
The doctor found that electrical stimulation of the lateral hypothalamus can force the rats to drink the bitter milk.
At the higher quinine levels they sometimes stopped drinking after the first lick, but returned again in a few seconds or stood at the tube making partial "approach-withdrawal" movements with the head and mouth.
As the concentration of quinine increased, the rats showed a decrease in drinking time, but this could be overcome by increasing the intensity of the stimulation.
At the highest stimulation intensity, the rats tolerated 8 times the concentration of quinine they did under 84-hour food deprivation.
Canadian researchers also showed that stimulation of the lateral hypothalamus induced intense drinking. (Mogenson and Stevenson 1966)
First, electrodes were placed in the lateral hypothalamus in rats, allowing them to self-stimulate by pressing a lever.
10 of the 12 rats actively self-stimulated, and made 4500-5500 lever presses during 30 minutes.
Next, the self-stimulation lever was removed, a water dispenser was installed, and the experimenter stimulated the same lateral hypothalamic electrode.
The rats had been provided with sufficient food and water.
They found that the four rats drank large amounts of water when stimulated. The number of laps ranged from 745 to 1,982 times for the 8-minute period, with total water intake ranging from 7.5 to 12.5 mL.
The remaining rats were unaffected by the stimulation and drank 0 mL of water.

Drinking behavior was stimulation-bound and ceased when electrical stimulation terminated.
Over the next hour, the drinking rats excreted as much as 4.0-8.5 mL of urine, whereas the no-drinking rats excreted only 0-0.5 mL of urine, confirming that water intake during brain stimulation was in excess of spontaneous need.
Then, the self-stimulation lever and the water dispenser were made available at the same time.
They found that the above four drinking rats alternated between pressing the lever and drinking water. The drinking behavior, although stimulation-bound, was now being controlled by the rats.
After each lever press, the rat drank until the electrical stimulation terminated and then pressed the lever to turn on the stimulation device again.
Ventral Tegmental Area
Electrical stimulation of the ventral tegmental area has also been shown to induce water intake in rats. (Waldbillig 1975)
The reactions were similar to those induced from the hypothalamus.
Each bout of drinking was limited to only a few licks, possibly because of interference from backing and crouching that was also elicited by the stimulation.
Unlike responses induced from the hypothalamus, drinking induced from the ventral tegmental area often persisted after the stimulation had ended.
Amygdala
Stimulation of the amygdala has been shown not only to induce water intake, but also to facilitate it. (Singer and Montgomery 1969)
In this experiment, instead of electrical stimulation, chemical stimulation was performed by injecting an acetylcholine analog, a neurotransmitter, into the cortical nucleus of the amygdala and the lateral hypothalamus (LH).
First, they confirmed that stimulation of the LH increased water intake in rats that had been given sufficient water.
They then found that additional stimulation of the cortical nucleus of the amygdala augmented this increase in water intake.
Amygdala stimulation alone increased water intake, but to a lesser extent than LH stimulation.
Amount of Water Intake
Amygdala (AMY) stimulation facilitated food intake induced by LH stimulation.
Other Primitive Behaviors
Lateral Hypothalamus
Electrical stimulation of the lateral hypothalamus in rats has been shown to produce gnawing and nest-building behavior. (Roberts and Carey 1965)
First, no spontaneous gnawing on the board in the absence of stimulation was observed.
Upon stimulation of some sites in the lateral hypothalamus, the rats engaged in exploratory movements, sniffing and looking around until they encountered the edge of the gnawing board.
Gripping the edge with their incisors, they braced their forepaws against an adjacent surface and pulled backward with their head and shoulders. As soon as a splinter or fragment was pulled loose, it was ejected from the mouth, and the rats returned to biting the edge.
The gnawing stopped promptly when the stimulation was terminated.
Even hungry rats stopped eating as soon as the stimulation was turned on, and began to gnaw on the board. While the board was present, they did not return to eating.
When the board was removed, the rats switched to exploratory movements and did not resume eating.
Upon stimulation of other sites, components of nest-building behavior were induced in rats, which included collecting paper strips in the mouth, shredding strips with repeated bites, pushing piles of strips with the snout, and burrowing.
Both gnawing and nest-building behavior were sometimes induced by stimulation of the same sites.
Electrical stimulation of the lateral hypothalamus in rats has also been shown to produce hoarding behavior. (Herberg and Blundell 1967)
Laboratory rats will not hoard food that is continuously available, but if they are placed on intermittent deprivation schedules for some days they will then begin to hoard at least as much while satiated as when they are hungry.
In the experiment, the cages were each provided with a partially enclosed home with nesting materials, and a water bottle.
They placed 100 food pellets and an equal number of similar wood blocks outside the home, and counted the number of food pellets carried into the home for 10 minutes.
Without electrical stimulation of the lateral hypothalamus, the satiated rats brought only about six pellets home.
In contrast, with stimulation, the satiated rats began immediate and sustained hoarding behavior, and brought a lot of pellets home.
The number of pellets hoarded was about 26, which was comparable to that during food deprivation.
The rats spent nearly half of each stimulation trial transporting the pellets, and in the remaining time they were able to eat no more than two pellets, far less than they collected.
Electrical stimulation of the lateral hypothalamus in rats has also been shown to produce object-carrying behavior. (Phillips et al. 1969)
Rodents carry objects in situations related to hoarding, nest building, and retrieving of young but may also carry items that don't have any obvious utility such as stones. The pack rat will even leave behind an object it had been carrying in favor of a more desirable shiny object.
In the experiment, an optical sensor was used to turn on electrical stimulation of the lateral hypothalamus when the rat entered one half of the cage and turn it off when the rat entered the other half.
When placed in the cage, the rats learned to move back and forth across the room to self-stimulate within a few minutes.
A pile of objects was then placed in the on-side of the cage. The objects included food pellets, dowel pins, wooden strips the same size as the pellets, and rubber erasers.
They found that the rats picked up the objects with their mouths and carried them to the off-side of the cage, where the rats deposited them as soon as the stimulation was turned off.
Objects were never picked up when the rats were not stimulated.
The rats selected the food pellets more often than the inedible objects, but an appreciable number of inedible objects were also carried. The 10-minute average was 40 food pellets and 26 inedible objects.
Ventral Tegmental Area
Electrical stimulation of the ventral tegmental area in rats has also been shown to produce gnawing behavior. (Waldbillig 1975)
Gnawing behavior obtained from the ventral tegmental area resembled that obtained from the lateral hypothalmus.
It consisted of gripping the edge of the wood with the incisors, bracing the forepaws against an adjacent surface, and pulling upward and backward with the head and shoulders.
As soon as a splinter or fragment was pulled loose, it was ejected from the mouth, and the rat returned to biting the edge.
Occasional bites were directed toward protruding metal edges, but did not persist.
Unlike responses induced from the hypothalamus, gnawing induced from the ventral tegmental area often persisted after the stimulation had ended.
Impulsivity, Hyperactivity
Nucleus Accumbens
Dutch researchers showed that electrical stimulation of the nucleus accumbens can increase impulsivity in rats. (Sesia et al. 2008)
In the experiment, the rats had to keep pushing a panel installed in the cage until a tone was presented.
Following this tone a lever was inserted, and by pressing the lever, the rat could obtain a food pellet.
The time between the pushing of the panel and the onset of the tone was randomly chosen in each trial from 0.6 to 1.5 seconds.
The rats that received electrical stimulation to the accumbal shell were unable to keep pushing the panel until the tone was presented, and more often failed to obtain the pellets.
This was interpreted as an increase in the impulsivity of the rats.
On the other hand, the rats that received electrical stimulation to the accumbal core showed the opposite tendency.
Ventral Tegmental Area
Electrical stimulation of the ventral tegmental area and nucleus accumbens has been shown to cause hyperactivity in cats. (Goldstein and Siegel 1980)
When electrical stimulation was applied to the ventral tegmental area, the cats exhibited searching head movements.
Increasing the stimulation intensity caused the cats to become hyperactive, with continuous walking, and frequent meowing.
When electrical stimulation was applied to the nucleus accumbens, the cats crouched slightly.
Increasing the stimulation intensity caused the cats to crouch pronouncedly, urinate, and become hyperactive markedly.
Following the termination of the stimulation, one cat displayed pre-seizure-like behavior, such as extreme hyperactivity, loud meowing, urination, and profuse salivation.
